Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis
- PMID: 21224324
- PMCID: PMC3019238
- DOI: 10.1136/bmj.c7086
Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis
Abstract
Objective: To analyse the available evidence on cardiovascular safety of non-steroidal anti-inflammatory drugs.
Design: Network meta-analysis.
Data sources: Bibliographic databases, conference proceedings, study registers, the Food and Drug Administration website, reference lists of relevant articles, and reports citing relevant articles through the Science Citation Index (last update July 2009). Manufacturers of celecoxib and lumiracoxib provided additional data.
Study selection: All large scale randomised controlled trials comparing any non-steroidal anti-inflammatory drug with other non-steroidal anti-inflammatory drugs or placebo. Two investigators independently assessed eligibility.
Data extraction: The primary outcome was myocardial infarction. Secondary outcomes included stroke, death from cardiovascular disease, and death from any cause. Two investigators independently extracted data.
Data synthesis: 31 trials in 116 429 patients with more than 115 000 patient years of follow-up were included. Patients were allocated to naproxen, ibuprofen, diclofenac, celecoxib, etoricoxib, rofecoxib, lumiracoxib, or placebo. Compared with placebo, rofecoxib was associated with the highest risk of myocardial infarction (rate ratio 2.12, 95% credibility interval 1.26 to 3.56), followed by lumiracoxib (2.00, 0.71 to 6.21). Ibuprofen was associated with the highest risk of stroke (3.36, 1.00 to 11.6), followed by diclofenac (2.86, 1.09 to 8.36). Etoricoxib (4.07, 1.23 to 15.7) and diclofenac (3.98, 1.48 to 12.7) were associated with the highest risk of cardiovascular death.
Conclusions: Although uncertainty remains, little evidence exists to suggest that any of the investigated drugs are safe in cardiovascular terms. Naproxen seemed least harmful. Cardiovascular risk needs to be taken into account when prescribing any non-steroidal anti-inflammatory drug.
Conflict of interest statement
Competing interests: All authors have completed the Unified Competing Interest form at
Figures
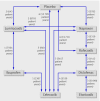
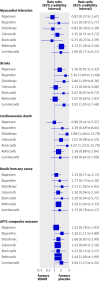

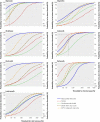
Comment in
-
Cardiovascular safety of NSAIDs.BMJ. 2011 Jan 11;342:c6618. doi: 10.1136/bmj.c6618. BMJ. 2011. PMID: 21224323 No abstract available.
-
Network meta-analysis. Results can be summarised in a simple figure.BMJ. 2011 Mar 23;342:d1555. doi: 10.1136/bmj.d1555. BMJ. 2011. PMID: 21430004 No abstract available.
-
NSAID use associated with increased cardiovascular risk and death, but naproxen appears to be the least harmful.Evid Based Med. 2011 Oct;16(5):142-3. doi: 10.1136/ebm11033. Epub 2011 Apr 3. Evid Based Med. 2011. PMID: 21460398 No abstract available.
-
Pain: The cardiovascular risks of NSAID use: which NSAID is the safest of them all?Nat Rev Rheumatol. 2011 Mar;7(3):129. doi: 10.1038/nrrheum.2011.9. Nat Rev Rheumatol. 2011. PMID: 21491645 No abstract available.
Similar articles
-
Cardiovascular safety of lumiracoxib: a meta-analysis of all randomized controlled trials > or =1 week and up to 1 year in duration of patients with osteoarthritis and rheumatoid arthritis.Clin Ther. 2005 Aug;27(8):1196-214. doi: 10.1016/j.clinthera.2005.07.019. Clin Ther. 2005. PMID: 16199245 Review.
-
Cardiovascular risk associated with celecoxib or etoricoxib: a meta-analysis of randomized controlled trials which adopted comparison with placebo or naproxen.Minerva Cardioangiol. 2014 Dec;62(6):437-48. Epub 2014 Jul 16. Minerva Cardioangiol. 2014. PMID: 25029569
-
Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), cardiovascular outcomes: randomised controlled trial.Lancet. 2004 Aug 21-27;364(9435):675-84. doi: 10.1016/S0140-6736(04)16894-3. Lancet. 2004. PMID: 15325832 Clinical Trial.
-
Non-steroidal anti-inflammatory drugs (NSAIDs) for chronic non-cancer pain in children and adolescents.Cochrane Database Syst Rev. 2017 Aug 2;8(8):CD012537. doi: 10.1002/14651858.CD012537.pub2. Cochrane Database Syst Rev. 2017. PMID: 28770976 Free PMC article. Review.
-
Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation.Health Technol Assess. 2008 Apr;12(11):1-278, iii. doi: 10.3310/hta12110. Health Technol Assess. 2008. PMID: 18405470 Review.
Cited by
-
Text Mining-Based Drug Discovery in Osteoarthritis.J Healthc Eng. 2021 Apr 14;2021:6674744. doi: 10.1155/2021/6674744. eCollection 2021. J Healthc Eng. 2021. PMID: 33953899 Free PMC article.
-
Prevalence of Gastrointestinal and Cardiovascular Risk in Patients with Degenerative Lumbar Spinal Disease.Clin Orthop Surg. 2020 Sep;12(3):343-352. doi: 10.4055/cios20021. Epub 2020 Aug 19. Clin Orthop Surg. 2020. PMID: 32904035 Free PMC article.
-
Cardiovascular risk.Br J Clin Pharmacol. 2012 Sep;74(3):396-410. doi: 10.1111/j.1365-2125.2012.04219.x. Br J Clin Pharmacol. 2012. PMID: 22348281 Free PMC article. Review.
-
Use of non-steroidal anti-inflammatory drugs that elevate cardiovascular risk: an examination of sales and essential medicines lists in low-, middle-, and high-income countries.PLoS Med. 2013;10(2):e1001388. doi: 10.1371/journal.pmed.1001388. Epub 2013 Feb 12. PLoS Med. 2013. PMID: 23424288 Free PMC article.
-
Management of chronic pain in the rheumatic diseases with insights for the clinician.Ther Adv Musculoskelet Dis. 2011 Aug;3(4):179-90. doi: 10.1177/1759720X11408999. Ther Adv Musculoskelet Dis. 2011. PMID: 22870477 Free PMC article.
References
-
- Dai C, Stafford RS, Alexander GC. National trends in cyclooxygenase-2 inhibitor use since market release: nonselective diffusion of a selectively cost-effective innovation. Arch Intern Med 2005;165:171-7. - PubMed
-
- Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA 2002;287:337-44. - PubMed
-
- Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med 2005;352:1092-102. - PubMed
-
- Juni P, Nartey L, Reichenbach S, Sterchi R, Dieppe PA, Egger M. Risk of cardiovascular events and rofecoxib: cumulative meta-analysis. Lancet 2004;364:2021-9. - PubMed
-
- McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. JAMA 2006;296:1633-44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
