Tunable, post-translational hydroxylation of collagen Domains in Escherichia coli
- PMID: 21210682
- PMCID: PMC3337207
- DOI: 10.1021/cb100298r
Tunable, post-translational hydroxylation of collagen Domains in Escherichia coli
Abstract
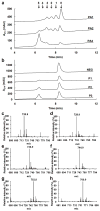
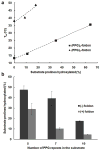
Prolyl 4-hydroxylases are ascorbate-dependent oxygenases that play key roles in a variety of eukaryotic biological processes including oxygen sensing, siRNA regulation, and collagen folding. They perform their functions by catalyzing the post-translational hydroxylation of specific proline residues on target proteins to form (2S,4R)-4-hydroxyproline. Thus far, the study of these post-translational modifications has been limited by the lack of a prokaryotic recombinant expression system for producing hydroxylated proteins. By introducing a biosynthetic shunt to produce ascorbate-like molecules in Eschericia coli cells that heterologously express human prolyl 4-hydroxylase (P4H), we have created a strain of E. coli that produces collagenous proteins with high levels of (2S,4R)-4-hydroxyproline. Using this new system, we have observed hydroxylation patterns indicative of a processive catalytic mode for P4H that is active even in the absence of ascorbate. Our results provide insights into P4H enzymology and create a foundation for better understanding how post-translational hydroxylation affects proteins.
Figures

Similar articles
-
Characterization of a second Arabidopsis thaliana prolyl 4-hydroxylase with distinct substrate specificity.J Biol Chem. 2005 Jan 14;280(2):1142-8. doi: 10.1074/jbc.M411109200. Epub 2004 Nov 4. J Biol Chem. 2005. PMID: 15528200
-
Collagen prolyl 4-hydroxylase isoenzymes I and II have sequence specificity towards different X-Pro-Gly triplets.Matrix Biol. 2024 Jan;125:73-87. doi: 10.1016/j.matbio.2023.12.001. Epub 2023 Dec 9. Matrix Biol. 2024. PMID: 38081527
-
Prolyl 4-hydroxylase.Crit Rev Biochem Mol Biol. 2010 Apr;45(2):106-24. doi: 10.3109/10409231003627991. Crit Rev Biochem Mol Biol. 2010. PMID: 20199358 Free PMC article. Review.
-
Role of prolyl hydroxylation in the molecular interactions of collagens.Essays Biochem. 2019 Sep 13;63(3):325-335. doi: 10.1042/EBC20180053. Print 2019 Sep 13. Essays Biochem. 2019. PMID: 31350381 Free PMC article. Review.
-
Hydroxylation of recombinant human collagen type I alpha 1 in transgenic maize co-expressed with a recombinant human prolyl 4-hydroxylase.BMC Biotechnol. 2011 Jun 24;11:69. doi: 10.1186/1472-6750-11-69. BMC Biotechnol. 2011. PMID: 21702901 Free PMC article.
Cited by
-
Multi-hierarchical self-assembly of a collagen mimetic peptide from triple helix to nanofibre and hydrogel.Nat Chem. 2011 Aug 28;3(10):821-8. doi: 10.1038/nchem.1123. Nat Chem. 2011. PMID: 21941256
-
Multiple site-selective insertions of noncanonical amino acids into sequence-repetitive polypeptides.Chembiochem. 2013 May 27;14(8):968-78. doi: 10.1002/cbic.201300069. Epub 2013 Apr 26. Chembiochem. 2013. PMID: 23625817 Free PMC article.
-
Hydroxylation of Human Type III Collagen Alpha Chain by Recombinant Coexpression with a Viral Prolyl 4-Hydroxylase in Escherichia coli.Protein J. 2017 Aug;36(4):322-331. doi: 10.1007/s10930-017-9723-0. Protein J. 2017. PMID: 28589291
-
Shining light on collagen: expressing collagen in plants.Tissue Eng Part A. 2013 Jul;19(13-14):1499-501. doi: 10.1089/ten.TEA.2013.0137. Epub 2013 Apr 30. Tissue Eng Part A. 2013. PMID: 23521064 Free PMC article.
-
Development and characterization of a eukaryotic expression system for human type II procollagen.BMC Biotechnol. 2015 Dec 15;15:112. doi: 10.1186/s12896-015-0228-7. BMC Biotechnol. 2015. PMID: 26666739 Free PMC article.
References
-
- Ozer A, Bruick RK. Non-heme dioxygenases: cellular sensors and regulators jelly rolled into one? Nat Chem Biol. 2007;3:144–153. - PubMed
-
- Loenarz C, Schofield CJ. Expanding chemical biology of 2-oxoglutarate oxygenases. Nat Chem Biol. 2008;4:152–156. - PubMed
-
- Berg RA, Prockop DJ. The thermal transition of a non-hydroxylated form of collagen. Evidence for a role for hydroxyproline in stabilizing the triple-helix of collagen. Biochem Biophys Res Commun. 1973;52:115–120. - PubMed
-
- Uitto J, Prockop DJ. Intracellular hydroxylation of non-helical protocollagen to form triple-helical procollagen and subsequent secretion of the molecule. Eur J Biochem. 1974;43:221–230. - PubMed
-
- Ramshaw JA, Shah NK, Brodsky B. Gly-X-Y tripeptide frequencies in collagen: a context for host-guest triple-helical peptides. J Struct Biol. 1998;122:86–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

