Anastral spindle assembly and γ-tubulin in Drosophila oocytes
- PMID: 21208439
- PMCID: PMC3022845
- DOI: 10.1186/1471-2121-12-1
Anastral spindle assembly and γ-tubulin in Drosophila oocytes
Abstract
Background: Anastral spindles assemble by a mechanism that involves microtubule nucleation and growth from chromatin. It is still uncertain whether γ-tubulin, a microtubule nucleator essential for mitotic spindle assembly and maintenance, plays a role. Not only is the requirement for γ-tubulin to form anastral Drosophila oocyte meiosis I spindles controversial, but its presence in oocyte meiosis I spindles has not been demonstrated and is uncertain.
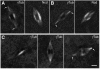
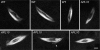
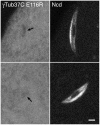
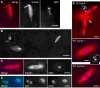
Results: We show, for the first time, using a bright GFP fusion protein and live imaging, that the Drosophila maternally-expressed γTub37C is present at low levels in oocyte meiosis I spindles. Despite this, we find that formation of bipolar meiosis I spindles does not require functional γTub37C, extending previous findings by others. Fluorescence photobleaching assays show rapid recovery of γTub37C in the meiosis I spindle, similar to the cytoplasm, indicating weak binding by γTub37C to spindles, and fits of a new, potentially more accurate model for fluorescence recovery yield kinetic parameters consistent with transient, diffusional binding.
Conclusions: The FRAP results, together with its mutant effects late in meiosis I, indicate that γTub37C may perform a role subsequent to metaphase I, rather than nucleating microtubules for meiosis I spindle formation. Weak binding to the meiosis I spindle could stabilize pre-existing microtubules or position γ-tubulin for function during meiosis II spindle assembly, which follows rapidly upon oocyte activation and completion of the meiosis I division.
Figures

Similar articles
-
Gamma-tubulin is required for bipolar spindle assembly and for proper kinetochore microtubule attachments during prometaphase I in Drosophila oocytes.PLoS Genet. 2011 Aug;7(8):e1002209. doi: 10.1371/journal.pgen.1002209. Epub 2011 Aug 11. PLoS Genet. 2011. PMID: 21852952 Free PMC article.
-
Oocyte Meiotic Spindle Assembly and Function.Curr Top Dev Biol. 2016;116:65-98. doi: 10.1016/bs.ctdb.2015.11.031. Epub 2016 Jan 23. Curr Top Dev Biol. 2016. PMID: 26970614 Free PMC article. Review.
-
Assembly and dynamics of an anastral:astral spindle: the meiosis II spindle of Drosophila oocytes.J Cell Sci. 1998 Sep;111 ( Pt 17):2487-95. doi: 10.1242/jcs.111.17.2487. J Cell Sci. 1998. PMID: 9701548
-
Assembly pathway of the anastral Drosophila oocyte meiosis I spindle.J Cell Sci. 2005 Apr 15;118(Pt 8):1745-55. doi: 10.1242/jcs.02304. Epub 2005 Mar 29. J Cell Sci. 2005. PMID: 15797926 Free PMC article.
-
Tubulin post-translational modifications in meiosis.Semin Cell Dev Biol. 2023 Mar 15;137:38-45. doi: 10.1016/j.semcdb.2021.11.014. Epub 2021 Nov 23. Semin Cell Dev Biol. 2023. PMID: 34836784 Free PMC article. Review.
Cited by
-
Gamma-tubulin is required for bipolar spindle assembly and for proper kinetochore microtubule attachments during prometaphase I in Drosophila oocytes.PLoS Genet. 2011 Aug;7(8):e1002209. doi: 10.1371/journal.pgen.1002209. Epub 2011 Aug 11. PLoS Genet. 2011. PMID: 21852952 Free PMC article.
-
Metaphase Spindle Assembly.Biology (Basel). 2017 Feb 3;6(1):8. doi: 10.3390/biology6010008. Biology (Basel). 2017. PMID: 28165376 Free PMC article. Review.
-
Oocyte Meiotic Spindle Assembly and Function.Curr Top Dev Biol. 2016;116:65-98. doi: 10.1016/bs.ctdb.2015.11.031. Epub 2016 Jan 23. Curr Top Dev Biol. 2016. PMID: 26970614 Free PMC article. Review.
-
Centrosomal and Non-Centrosomal Microtubule-Organizing Centers (MTOCs) in Drosophila melanogaster.Cells. 2018 Aug 28;7(9):121. doi: 10.3390/cells7090121. Cells. 2018. PMID: 30154378 Free PMC article. Review.
References
-
- Liu B, Marc J, Joshi HC, Palevitz BA. A γ-tubulin-related protein associated with the microtubule arrays of higher plants in a cell cycle-dependent manner. J Cell Sci. 1993;104:1217–1228. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

