Role of sphingomyelin synthase in controlling the antimicrobial activity of neutrophils against Cryptococcus neoformans
- PMID: 21203393
- PMCID: PMC3011003
- DOI: 10.1371/journal.pone.0015587
Role of sphingomyelin synthase in controlling the antimicrobial activity of neutrophils against Cryptococcus neoformans
Abstract
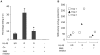
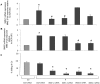
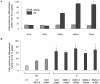
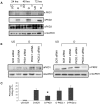
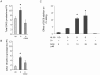
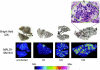
The key host cellular pathway(s) necessary to control the infection caused by inhalation of the environmental fungal pathogen Cryptococcus neoformans are still largely unknown. Here we have identified that the sphingolipid pathway in neutrophils is required for them to exert their killing activity on the fungus. In particular, using both pharmacological and genetic approaches, we show that inhibition of sphingomyelin synthase (SMS) activity profoundly impairs the killing ability of neutrophils by preventing the extracellular release of an antifungal factor(s). We next found that inhibition of protein kinase D (PKD), which controls vesicular sorting and secretion and is regulated by diacylglycerol (DAG) produced by SMS, totally blocks the extracellular killing activity of neutrophils against C. neoformans. The expression of SMS genes, SMS activity and the levels of the lipids regulated by SMS (namely sphingomyelin (SM) and DAG) are up-regulated during neutrophil differentiation. Finally, tissue imaging of lungs infected with C. neoformans using matrix-assisted laser desorption-ionization mass spectrometry (MALDI-MS), revealed that specific SM species are associated with neutrophil infiltration at the site of the infection. This study establishes a key role for SMS in the regulation of the killing activity of neutrophils against C. neoformans through a DAG-PKD dependent mechanism, and provides, for the first time, new insights into the protective role of host sphingolipids against a fungal infection.
Conflict of interest statement
Figures
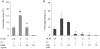
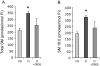

Similar articles
-
Cryptococcus neoformans modulates extracellular killing by neutrophils.Front Microbiol. 2011 Sep 21;2:193. doi: 10.3389/fmicb.2011.00193. eCollection 2011. Front Microbiol. 2011. PMID: 21960987 Free PMC article.
-
The sphingolipid pathway regulates Pkc1 through the formation of diacylglycerol in Cryptococcus neoformans.J Biol Chem. 2004 May 14;279(20):21144-53. doi: 10.1074/jbc.M312995200. Epub 2004 Mar 10. J Biol Chem. 2004. PMID: 15014071
-
Sphingomyelin synthase, a potential regulator of intracellular levels of ceramide and diacylglycerol during SV40 transformation. Does sphingomyelin synthase account for the putative phosphatidylcholine-specific phospholipase C?J Biol Chem. 1998 Jun 5;273(23):14550-9. doi: 10.1074/jbc.273.23.14550. J Biol Chem. 1998. PMID: 9603970
-
Combatting the evolution of antifungal resistance in Cryptococcus neoformans.Mol Microbiol. 2020 Nov;114(5):721-734. doi: 10.1111/mmi.14565. Epub 2020 Jul 22. Mol Microbiol. 2020. PMID: 32697029 Review.
-
[Melanin and its role on the virulence of Cryptococcus neoformans].Mikrobiyol Bul. 2010 Jul;44(3):519-26. Mikrobiyol Bul. 2010. PMID: 21064003 Review. Turkish.
Cited by
-
Kidney glycosphingolipids are elevated early in diabetic nephropathy and mediate hypertrophy of mesangial cells.Am J Physiol Renal Physiol. 2015 Aug 1;309(3):F204-15. doi: 10.1152/ajprenal.00150.2015. Epub 2015 Jun 3. Am J Physiol Renal Physiol. 2015. PMID: 26041445 Free PMC article.
-
Sphingomyelin synthases regulate protein trafficking and secretion.PLoS One. 2011;6(9):e23644. doi: 10.1371/journal.pone.0023644. Epub 2011 Sep 27. PLoS One. 2011. PMID: 21980337 Free PMC article.
-
Gene expression during the generation and activation of mouse neutrophils: implication of novel functional and regulatory pathways.PLoS One. 2014 Oct 3;9(10):e108553. doi: 10.1371/journal.pone.0108553. eCollection 2014. PLoS One. 2014. PMID: 25279834 Free PMC article.
-
Induction of brain microvascular endothelial cell urokinase expression by Cryptococcus neoformans facilitates blood-brain barrier invasion.PLoS One. 2012;7(11):e49402. doi: 10.1371/journal.pone.0049402. Epub 2012 Nov 8. PLoS One. 2012. PMID: 23145170 Free PMC article.
-
Imaging with mass spectrometry, the next frontier in sphingolipid research? A discussion on where we stand and the possibilities ahead.Chem Phys Lipids. 2019 Mar;219:1-14. doi: 10.1016/j.chemphyslip.2019.01.001. Epub 2019 Jan 11. Chem Phys Lipids. 2019. PMID: 30641043 Free PMC article. Review.
References
-
- Sanfelice F. Contributo alla morfologia e biologia dei blastomiceti che si sviluppano nei succhi di alcuni frutti. Ann Igien. 1894;4:463–495.
-
- Busse O. Uber parasitare zelleinschlusse und ihre zuchtung. Zentralbl Bakteriol. 1894;16:175–180.
-
- Buschke A. Uber eine durch Coccidien Hervergerufene Krankheit des menschen. Dtsch Med Wochenschr. 1895;21:14.
-
- Casadevall A, Perfect JR. Epidemiology. In: Casadevall A, Perfect JR, editors. Cryptococcus neoformans. Washington, DC: ASM; 1998. pp. 351–380.
-
- Fraser JA, Giles SS, Wenink EC, Geunes-Boyer SG, Wright JR, et al. Same-sex mating and the origin of the Vancouver Island Cryptococcus gattii outbreak. Nature. 2005;437:1360–1364. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

