Lack of α8 integrin leads to morphological changes in renal mesangial cells, but not in vascular smooth muscle cells
- PMID: 21194485
- PMCID: PMC3022721
- DOI: 10.1186/1471-2121-11-102
Lack of α8 integrin leads to morphological changes in renal mesangial cells, but not in vascular smooth muscle cells
Abstract
Background: Extracellular matrix receptors of the integrin family are known to regulate cell adhesion, shape and functions. The α8 integrin chain is expressed in glomerular mesangial cells and in vascular smooth muscle cells. Mice deficient for α8 integrin have structural alterations in glomeruli but not in renal arteries. For this reason we hypothesized that mesangial cells and vascular smooth muscle cells differ in their respective capacity to compensate for the lack of α8 integrin.
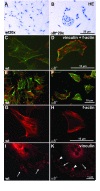
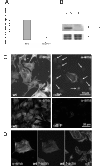
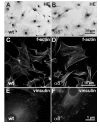
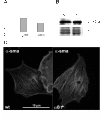
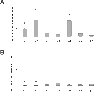
Results: Wild type and α8 integrin-deficient mesangial cells varied markedly in cell morphology and expression or localization of cytoskeletal molecules. In α8 integrin-deficient mesangial cells α-smooth muscle actin and CTGF were downregulated. In contrast, there were no comparable differences between α8 integrin-deficient and wild type vascular smooth muscle cells. Expression patterns of integrins were altered in α8 integrin-deficient mesangial cells compared to wild type mesangial cells, displaying a prominent overexpression of α2 and α6 integrins, while expression patterns of the these integrins were not different between wild type and α8 integrin-deficient vascular smooth muscle cells, respectively. Cell proliferation was augmented in α8 integrin-deficient mesangial cells, but not in vascular smooth muscle cells, compared to wild type cells.
Conclusions: Our findings suggest that α8 integrin deficiency has differential effects in mesangial cells and vascular smooth muscle cells. While the phenotype of vascular smooth muscle cells lacking α8 integrin is not altered, mesangial cells lacking α8 integrin differ considerably from wild type mesangial cells which might be a consequence of compensatory changes in the expression patterns of other integrins. This could result in glomerular changes in α8 integrin-deficient mice, while the vasculature is not affected in these mice.
Figures




Similar articles
-
Glomerular and renal vascular structural changes in alpha8 integrin-deficient mice.J Am Soc Nephrol. 2003 Sep;14(9):2288-96. doi: 10.1097/01.asn.0000082999.46030.fe. J Am Soc Nephrol. 2003. PMID: 12937305
-
Fibrillin-1 and alpha8 integrin are co-expressed in the glomerulus and interact to convey adhesion of mesangial cells.Cell Adh Migr. 2014;8(4):389-95. doi: 10.4161/cam.28988. Cell Adh Migr. 2014. PMID: 25482639 Free PMC article.
-
Role of alpha8 integrin in mesangial cell adhesion, migration, and proliferation.Kidney Int. 2003 Jul;64(1):119-27. doi: 10.1046/j.1523-1755.2003.00057.x. Kidney Int. 2003. PMID: 12787402
-
Lessons in congenital and acquired renal disease from alpha8 integrin mutant mice.Pediatr Nephrol. 2002 Nov;17(11):882-8. doi: 10.1007/s00467-002-0950-y. Epub 2002 Aug 16. Pediatr Nephrol. 2002. PMID: 12432427 Review.
-
Tensegrin in context: Dual role of α8 integrin in the migration of different cell types.Cell Adh Migr. 2010 Oct-Dec;4(4):485-90. doi: 10.4161/cam.4.4.12403. Cell Adh Migr. 2010. PMID: 20543583 Free PMC article. Review.
Cited by
-
A role for the alpha-8 integrin chain (itga8) in glomerular homeostasis of the kidney.Mol Cell Pediatr. 2020 Oct 1;7(1):13. doi: 10.1186/s40348-020-00105-5. Mol Cell Pediatr. 2020. PMID: 33000355 Free PMC article. Review.
-
Glomerular mesangial cell recruitment and function require the co-receptor neuropilin-1.Am J Physiol Renal Physiol. 2017 Dec 1;313(6):F1232-F1242. doi: 10.1152/ajprenal.00311.2017. Epub 2017 Aug 23. Am J Physiol Renal Physiol. 2017. PMID: 28835419 Free PMC article.
-
Nephronectin Regulates Mesangial Cell Adhesion and Behavior in Glomeruli.J Am Soc Nephrol. 2018 Apr;29(4):1128-1140. doi: 10.1681/ASN.2017070752. Epub 2018 Jan 15. J Am Soc Nephrol. 2018. PMID: 29335243 Free PMC article.
-
Digital spatial profiling of segmental outflow regions in trabecular meshwork reveals a role for ADAM15.PLoS One. 2024 Feb 23;19(2):e0298802. doi: 10.1371/journal.pone.0298802. eCollection 2024. PLoS One. 2024. PMID: 38394161 Free PMC article.
-
Role of integrin alpha8 in murine model of lung fibrosis.PLoS One. 2018 May 29;13(5):e0197937. doi: 10.1371/journal.pone.0197937. eCollection 2018. PLoS One. 2018. PMID: 29813125 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

