Adjuvant-free immunization with hemagglutinin-Fc fusion proteins as an approach to influenza vaccines
- PMID: 21191017
- PMCID: PMC3067967
- DOI: 10.1128/JVI.01241-10
Adjuvant-free immunization with hemagglutinin-Fc fusion proteins as an approach to influenza vaccines
Abstract
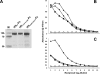
The hemagglutinins (HAs) of human H1 and H3 influenza viruses and avian H5 influenza virus were produced as recombinant fusion proteins with the human immunoglobulin Fc domain. Recombinant HA-human immunoglobulin Fc domain (HA-HuFc) proteins were secreted from baculovirus-infected insect cells as glycosylated oligomer HAs of the anticipated molecular mass, agglutinated red blood cells, were purified on protein A, and were used to immunize mice in the absence of adjuvant. Immunogenicity was demonstrated for all subtypes, with the serum samples demonstrating subtype-specific hemagglutination inhibition, epitope specificity similar to that seen with virus infection, and neutralization. HuFc-tagged HAs are potential candidates for gene-to-vaccine approaches to influenza vaccination.
Figures
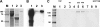
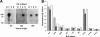

Similar articles
-
Recombinant influenza H7 hemagglutinins induce lower neutralizing antibody titers in mice than do seasonal hemagglutinins.Influenza Other Respir Viruses. 2014 Nov;8(6):628-35. doi: 10.1111/irv.12285. Epub 2014 Sep 12. Influenza Other Respir Viruses. 2014. PMID: 25213778 Free PMC article.
-
Immunization with influenza A virus hemagglutinin and neuraminidase produced in recombinant baculovirus results in a balanced and broadened immune response superior to conventional vaccine.Vaccine. 1999 Apr 9;17(15-16):2073-80. doi: 10.1016/s0264-410x(98)00413-7. Vaccine. 1999. PMID: 10217609
-
Comparative immunogenicity of recombinant adenovirus-vectored vaccines expressing different forms of hemagglutinin (HA) proteins from the H5 serotype of influenza A viruses in mice.Virus Res. 2011 Jan;155(1):156-62. doi: 10.1016/j.virusres.2010.09.014. Epub 2010 Sep 29. Virus Res. 2011. PMID: 20883733
-
Immunization with baculovirus displayed H6 hemagglutinin vaccine protects mice against lethal H6 influenza virus challenge.Antiviral Res. 2014 Sep;109:42-53. doi: 10.1016/j.antiviral.2014.06.002. Epub 2014 Jun 25. Antiviral Res. 2014. PMID: 24973759
-
Production of a novel influenza vaccine using insect cells: protection against drifted strains.Influenza Other Respir Viruses. 2007 Jan;1(1):35-40. doi: 10.1111/j.1750-2659.2006.00007.x. Influenza Other Respir Viruses. 2007. PMID: 19453478 Free PMC article. Review.
Cited by
-
Intranasal vaccination of recombinant H5N1 HA1 proteins fused with foldon and Fc induces strong mucosal immune responses with neutralizing activity: Implication for developing novel mucosal influenza vaccines.Hum Vaccin Immunother. 2015;11(12):2831-8. doi: 10.1080/21645515.2015.1074363. Hum Vaccin Immunother. 2015. PMID: 26260706 Free PMC article.
-
Engineering a stable CHO cell line for the expression of a MERS-coronavirus vaccine antigen.Vaccine. 2018 Mar 27;36(14):1853-1862. doi: 10.1016/j.vaccine.2018.02.065. Epub 2018 Feb 26. Vaccine. 2018. PMID: 29496347 Free PMC article.
-
Preclinical assessment of a recombinant RBD-Fc fusion protein as SARS-CoV-2 candidate vaccine.Eur J Microbiol Immunol (Bp). 2024 May 16;14(3):228-242. doi: 10.1556/1886.2024.00045. Print 2024 Sep 11. Eur J Microbiol Immunol (Bp). 2024. PMID: 38753442 Free PMC article.
-
CFP10: mFcγ2 as a novel tuberculosis vaccine candidate increases immune response in mouse.Iran J Basic Med Sci. 2017 Feb;20(2):122-130. doi: 10.22038/ijbms.2017.8231. Iran J Basic Med Sci. 2017. PMID: 28293387 Free PMC article.
-
Development of a recombinant vaccine containing a spike S1-Fc fusion protein induced protection against MERS-CoV in human DPP4 knockin transgenic mice.J Virol Methods. 2022 Jan;299:114347. doi: 10.1016/j.jviromet.2021.114347. Epub 2021 Oct 30. J Virol Methods. 2022. PMID: 34728273 Free PMC article.
References
-
- Barclay, W. S., et al. 2007. Probing the receptor interactions of an H5 avian influenza virus using a baculovirus expression system and functionalised poly(acrylic acid) ligands. Bioorg. Med. Chem. 15:4038-4047. - PubMed
-
- Bright, R. A., et al. 2007. Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin. Vaccine 25:3871-3878. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

