Observing selected domains in multi-domain proteins via sortase-mediated ligation and NMR spectroscopy
- PMID: 21188472
- PMCID: PMC3111449
- DOI: 10.1007/s10858-010-9464-2
Observing selected domains in multi-domain proteins via sortase-mediated ligation and NMR spectroscopy
Abstract
NMR spectroscopy has distinct advantages for providing insight into protein structures, but faces significant resolution challenges as protein size increases. To alleviate such resonance overlap issues, the ability to produce segmentally labeled proteins is beneficial. Here we show that the S. aureus transpeptidase sortase A can be used to catalyze the ligation of two separately expressed domains of the same protein, MecA (B. subtilis). The yield of purified, segmentally labeled MecA protein conjugate is approximately 40%. The resultant HSQC spectrum obtained from this domain-labeled conjugate demonstrates successful application of sortase A for segmental labeling of multi-domain proteins for solution NMR study.
Figures
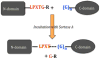

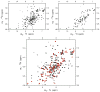
Similar articles
-
Efficient segmental isotope labeling of multi-domain proteins using Sortase A.J Biomol NMR. 2015 Sep;63(1):1-8. doi: 10.1007/s10858-015-9981-0. Epub 2015 Aug 30. J Biomol NMR. 2015. PMID: 26319988
-
Attachment of an NMR-invisible solubility enhancement tag using a sortase-mediated protein ligation method.J Biomol NMR. 2009 Mar;43(3):145-50. doi: 10.1007/s10858-008-9296-5. Epub 2009 Jan 13. J Biomol NMR. 2009. PMID: 19140010
-
Domain selective labeling for NMR studies of multidomain proteins by domain ligation using highly active sortase A.Biochim Biophys Acta Gen Subj. 2020 Feb;1864(2):129419. doi: 10.1016/j.bbagen.2019.129419. Epub 2019 Aug 23. Biochim Biophys Acta Gen Subj. 2020. PMID: 31449838
-
Making and breaking peptide bonds: protein engineering using sortase.Angew Chem Int Ed Engl. 2011 May 23;50(22):5024-32. doi: 10.1002/anie.201008267. Epub 2011 Apr 27. Angew Chem Int Ed Engl. 2011. PMID: 21538739 Review.
-
Sortase-mediated backbone cyclization of proteins and peptides.Biol Chem. 2015 Apr;396(4):283-93. doi: 10.1515/hsz-2014-0260. Biol Chem. 2015. PMID: 25581753 Review.
Cited by
-
Function and dynamics of the intrinsically disordered carboxyl terminus of β2 adrenergic receptor.Nat Commun. 2023 Apr 10;14(1):2005. doi: 10.1038/s41467-023-37233-1. Nat Commun. 2023. PMID: 37037825 Free PMC article.
-
Practical aspects of NMR signal assignment in larger and challenging proteins.Prog Nucl Magn Reson Spectrosc. 2014 Apr;78:47-75. doi: 10.1016/j.pnmrs.2013.12.001. Epub 2013 Dec 15. Prog Nucl Magn Reson Spectrosc. 2014. PMID: 24534088 Free PMC article. Review.
-
Biomolecular condensates amplify mRNA decapping by biasing enzyme conformation.Nat Chem Biol. 2021 May;17(5):615-623. doi: 10.1038/s41589-021-00774-x. Epub 2021 Mar 25. Nat Chem Biol. 2021. PMID: 33767388 Free PMC article.
-
Challenges in the use of sortase and other peptide ligases for site-specific protein modification.Chem Soc Rev. 2022 May 23;51(10):4121-4145. doi: 10.1039/d0cs01148g. Chem Soc Rev. 2022. PMID: 35510539 Free PMC article. Review.
-
The dynamic duo: combining NMR and small angle scattering in structural biology.Protein Sci. 2014 Jun;23(6):669-82. doi: 10.1002/pro.2467. Epub 2014 Apr 17. Protein Sci. 2014. PMID: 24687405 Free PMC article. Review.
References
-
- Clow F, Fraser JD, Proft T. Immobilization of proteins to biacore sensor chips using Staphylococcus aureus sortase A. Biotechnol Lett. 2008;30:1603–1647. - PubMed
-
- Dawson PE, Muir TW, Clark-Lewis I, Kent SB. Synthesis of proteins by native chemical ligation. Science. 1994;266:776–779. - PubMed
-
- Eismann T, Huber N, Shin T, Kuboki S, Galloway E, Wyder M, Edwards MJ, Greis KD, Shertzer HG, Fisher AB, Lentsch AB. Peroxiredoxin-6 protects against mitochondrial dysfunction and liver injury during ischemia/reperfusion in mice. Am J Physiol Gastrointest Liver Physiol. 2008;296:G266–G274. - PMC - PubMed
-
- Iwai H, Züger S. Protein ligation: application in NMR studies of proteins. Biotechnol Genet Eng Rev. 2007;24:129–146. - PubMed
-
- Kobashigawa Y, Kumeta H, Ogura K, Inagaki F. Attachment of an NMR-invisible solubility enhancement tag using a sortase-mediated protein ligation method. J Biomol NMR. 2009;43:145–150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

