The TNFR family members OX40 and CD27 link viral virulence to protective T cell vaccines in mice
- PMID: 21183789
- PMCID: PMC3007137
- DOI: 10.1172/JCI42056
The TNFR family members OX40 and CD27 link viral virulence to protective T cell vaccines in mice
Abstract
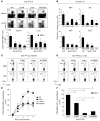
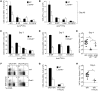
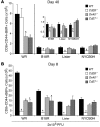
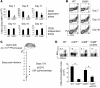
Induction of CD8+ T cell immunity is a key characteristic of an effective vaccine. For safety reasons, human vaccination strategies largely use attenuated nonreplicating or weakly replicating poxvirus-based vectors, but these often elicit poor CD8+ T cell immunity and might not result in optimal protection. Recent studies have suggested that virulence is directly linked to immunogenicity, but the molecular mechanisms underlying optimal CD8+ T cell responses remain to be defined. Here, using natural and recombinant vaccinia virus (VACV) strains, we have shown in mice that VACV strains of differing virulence induce distinct levels of T cell memory because of the differential use of TNF receptor (TNFR) family costimulatory receptors. With strongly replicating (i.e., virulent) VACV, the TNFR family costimulatory receptors OX40 (also known as CD134) and CD27 were engaged and promoted the generation of high numbers of memory CD8+ T cells, which protected against a lethal virus challenge in the absence of other mechanisms, including antibody and help from CD4+ T cells. In contrast, weakly replicating (i.e., low-virulence) VACV strains were poor at eliciting protective CD8+ T cell memory, as only the Ig family costimulatory receptor CD28 was engaged, and not OX40 or CD27. Our results suggest that the virulence of a virus dictates costimulatory receptor usage to determine the level of protective CD8+ T cell immunity.
Figures




Comment in
-
A stimulating way to improve T cell responses to poxvirus-vectored vaccines.J Clin Invest. 2011 Jan;121(1):19-21. doi: 10.1172/JCI45726. Epub 2010 Dec 22. J Clin Invest. 2011. PMID: 21183782 Free PMC article.
Similar articles
-
Targeting OX40 promotes lung-resident memory CD8 T cell populations that protect against respiratory poxvirus infection.J Virol. 2011 Sep;85(17):9051-9. doi: 10.1128/JVI.00619-11. Epub 2011 Jun 29. J Virol. 2011. PMID: 21715499 Free PMC article.
-
OX40 drives protective vaccinia virus-specific CD8 T cells.J Immunol. 2008 Dec 1;181(11):7969-76. doi: 10.4049/jimmunol.181.11.7969. J Immunol. 2008. PMID: 19017988 Free PMC article.
-
Dispensable role for 4-1BB and 4-1BBL in development of vaccinia virus-specific CD8 T cells.Immunol Lett. 2012 Jan 30;141(2):220-6. doi: 10.1016/j.imlet.2011.10.008. Epub 2011 Oct 20. Immunol Lett. 2012. PMID: 22037570 Free PMC article.
-
OX40:OX40L axis: emerging targets for improving poxvirus-based CD8(+) T-cell vaccines against respiratory viruses.Immunol Rev. 2011 Nov;244(1):149-68. doi: 10.1111/j.1600-065X.2011.01062.x. Immunol Rev. 2011. PMID: 22017437 Free PMC article. Review.
-
Costimulation of T cells by OX40, 4-1BB, and CD27.Cytokine Growth Factor Rev. 2003 Jun-Aug;14(3-4):265-73. doi: 10.1016/s1359-6101(03)00025-x. Cytokine Growth Factor Rev. 2003. PMID: 12787564 Review.
Cited by
-
CD27-CD70 costimulation controls T cell immunity during acute and persistent cytomegalovirus infection.J Virol. 2013 Jun;87(12):6851-65. doi: 10.1128/JVI.03305-12. Epub 2013 Apr 10. J Virol. 2013. PMID: 23576505 Free PMC article.
-
Lack of B Lymphocytes Enhances CD8 T Cell-Mediated Resistance against Respiratory Viral Infection but Compromises Memory Cell Formation.J Virol. 2020 Jan 17;94(3):e01877-19. doi: 10.1128/JVI.01877-19. Print 2020 Jan 17. J Virol. 2020. PMID: 31723023 Free PMC article.
-
Batf3-Dependent Dendritic Cells Promote Optimal CD8 T Cell Responses against Respiratory Poxvirus Infection.J Virol. 2018 Jul 31;92(16):e00495-18. doi: 10.1128/JVI.00495-18. Print 2018 Aug 15. J Virol. 2018. PMID: 29875235 Free PMC article.
-
Targeting OX40 promotes lung-resident memory CD8 T cell populations that protect against respiratory poxvirus infection.J Virol. 2011 Sep;85(17):9051-9. doi: 10.1128/JVI.00619-11. Epub 2011 Jun 29. J Virol. 2011. PMID: 21715499 Free PMC article.
-
CD8 T cells use IFN-γ to protect against the lethal effects of a respiratory poxvirus infection.J Immunol. 2014 Jun 1;192(11):5415-25. doi: 10.4049/jimmunol.1400256. Epub 2014 Apr 18. J Immunol. 2014. PMID: 24748494 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

