Reaction pathway and free energy profile for butyrylcholinesterase-catalyzed hydrolysis of acetylcholine
- PMID: 21175195
- PMCID: PMC3033463
- DOI: 10.1021/jp110709a
Reaction pathway and free energy profile for butyrylcholinesterase-catalyzed hydrolysis of acetylcholine
Abstract
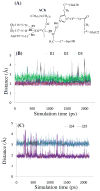
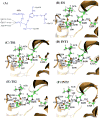
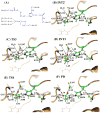
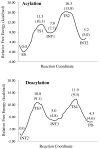
A catalytic mechanism for the butyrylcholinesterase (BChE)-catalyzed hydrolysis of acetylcholine (ACh) has been studied by performing pseudobond first-principles quantum mechanical/molecular mechanical-free energy calculations on both acylation and deacylation of BChE. It has been shown that the acylation with ACh includes two reaction steps, including nucleophilic attack on the carbonyl carbon of ACh and dissociation of choline ester. The deacylation stage includes nucleophilic attack of a water molecule on the carboxyl carbon of the substrate and dissociation between the carboxyl carbon of the substrate and the hydroxyl oxygen of the Ser198 side chain. Notably, despite the fact that acetylcholinesterase (AChE) and BChE are very similar enzymes, the acylation of BChE with ACh is rate-determining, which is remarkably different from the AChE-catalyzed hydrolysis of ACh, in which the deacylation is rate-determining. The computational prediction is consistent with available experimental kinetic data. The overall free energy barrier calculated for BChE-catalyzed hydrolysis of ACh is 13.8 kcal/mol, which is in good agreement with the experimentally derived activation free energy of 13.3 kcal/mol.
Figures

Similar articles
-
Reaction pathway and free energy profiles for butyrylcholinesterase-catalyzed hydrolysis of acetylthiocholine.Biochemistry. 2012 Feb 14;51(6):1297-305. doi: 10.1021/bi201786s. Epub 2012 Feb 3. Biochemistry. 2012. PMID: 22304234 Free PMC article.
-
Fundamental reaction pathway and free energy profile for butyrylcholinesterase-catalyzed hydrolysis of heroin.Biochemistry. 2013 Sep 17;52(37):6467-79. doi: 10.1021/bi400709v. Epub 2013 Aug 30. Biochemistry. 2013. PMID: 23992153 Free PMC article.
-
Fundamental reaction mechanism and free energy profile for (-)-cocaine hydrolysis catalyzed by cocaine esterase.J Am Chem Soc. 2009 Aug 26;131(33):11964-75. doi: 10.1021/ja903990p. J Am Chem Soc. 2009. PMID: 19642701 Free PMC article.
-
Optical imaging probes for selective detection of butyrylcholinesterase.J Mater Chem B. 2024 Jan 31;12(5):1149-1167. doi: 10.1039/d3tb02468g. J Mater Chem B. 2024. PMID: 38196348 Review.
-
Substrate deacylation mechanisms of serine-beta-lactamases.Biol Pharm Bull. 2006 Nov;29(11):2151-9. doi: 10.1248/bpb.29.2151. Biol Pharm Bull. 2006. PMID: 17077507 Review.
Cited by
-
Application of Computational Biology and Artificial Intelligence in Drug Design.Int J Mol Sci. 2022 Nov 5;23(21):13568. doi: 10.3390/ijms232113568. Int J Mol Sci. 2022. PMID: 36362355 Free PMC article. Review.
-
Catalytic Hydrolysis Mechanism of Cocaine by Human Carboxylesterase 1: An Orthoester Intermediate Slows Down the Reaction.Molecules. 2019 Nov 9;24(22):4057. doi: 10.3390/molecules24224057. Molecules. 2019. PMID: 31717501 Free PMC article.
-
Binding free energies for nicotine analogs inhibiting cytochrome P450 2A6 by a combined use of molecular dynamics simulations and QM/MM-PBSA calculations.Bioorg Med Chem. 2014 Apr 1;22(7):2149-56. doi: 10.1016/j.bmc.2014.02.037. Epub 2014 Mar 3. Bioorg Med Chem. 2014. PMID: 24631364 Free PMC article.
-
Fundamental reaction pathway and free energy profile of proteasome inhibition by syringolin A (SylA).Org Biomol Chem. 2015 Jun 28;13(24):6857-65. doi: 10.1039/c5ob00737b. Org Biomol Chem. 2015. PMID: 26018983 Free PMC article.
-
Catalytic Reaction Mechanism for Drug Metabolism in Human Carboxylesterase-1: Cocaine Hydrolysis Pathway.Mol Pharm. 2018 Sep 4;15(9):3871-3880. doi: 10.1021/acs.molpharmaceut.8b00354. Epub 2018 Aug 10. Mol Pharm. 2018. PMID: 30095924 Free PMC article.
References
-
- Fuxreiter M, Warshel A. J Am Chem Soc. 1998;120(1):183–194.
-
- Boeck AT, Schopfer LM, Lockridge O. Biochem Pharmacol. 2002;63(12):2101–2110. - PubMed
-
- Masson P, Froment MT, Gillon E, Nachon F, Lockridge O, Schopfer LM. FEBS J. 2008;275(10):2617–2631. - PubMed
-
- Mesulam MM, Guillozet A, Shaw P, Levey A, Duysen EG, Lockridge O. Neuroscience. 2002;110(4):627–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

