Development of FRET assay into quantitative and high-throughput screening technology platforms for protein-protein interactions
- PMID: 21174150
- PMCID: PMC3069323
- DOI: 10.1007/s10439-010-0225-x
Development of FRET assay into quantitative and high-throughput screening technology platforms for protein-protein interactions
Abstract
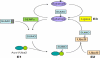
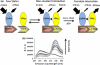
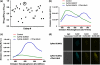
Förster resonance energy transfer (FRET) technology has been widely used in biological and biomedical research and is a very powerful tool in elucidating protein interactions in many cellular processes. Ubiquitination and SUMOylation are multi-step cascade reactions, involving multiple enzymes and protein-protein interactions. Here we report the development of dissociation constant (K (d)) determination for protein-protein interaction and cell-based high-throughput screening (HTS) assay in SUMOylation cascade using FRET technology. These developments are based on steady state and high efficiency of fluorescent energy transfer between CyPet and YPet fused with SUMO1 and Ubc9, respectively. The developments in theoretical and experimental procedures for protein interaction K (d) determination and cell-based HTS provide novel tools in affinity measurement and protein interaction inhibitor screening. The K (d) determined by FRET between SUMO1 and Ubc9 is compatible with those determined with other traditional approaches, such as isothermal titration calorimetry (ITC) and surface plasmon resonance (SPR). The FRET-based HTS is pioneer in cell-based HTS. Both K (d) determination and cell-based HTS, carried out in 384-well plate format, provide powerful tools for large-scale and high-throughput applications.
Figures
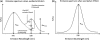
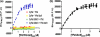
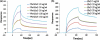
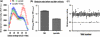
Similar articles
-
An in vitro Förster resonance energy transfer-based high-throughput screening assay for inhibitors of protein-protein interactions in SUMOylation pathway.Assay Drug Dev Technol. 2012 Aug;10(4):336-43. doi: 10.1089/adt.2011.0394. Epub 2011 Dec 22. Assay Drug Dev Technol. 2012. PMID: 22192309 Free PMC article.
-
Protein interaction affinity determination by quantitative FRET technology.Biotechnol Bioeng. 2012 Nov;109(11):2875-83. doi: 10.1002/bit.24564. Epub 2012 Jun 18. Biotechnol Bioeng. 2012. PMID: 22711490
-
Quantitative Förster resonance energy transfer analysis for kinetic determinations of SUMO-specific protease.Anal Biochem. 2012 Mar 1;422(1):14-21. doi: 10.1016/j.ab.2011.12.019. Epub 2011 Dec 24. Anal Biochem. 2012. PMID: 22244808
-
Quantitative FRET (qFRET) Technology for the Determination of Protein-Protein Interaction Affinity in Solution.Molecules. 2021 Oct 20;26(21):6339. doi: 10.3390/molecules26216339. Molecules. 2021. PMID: 34770748 Free PMC article. Review.
-
A new trend to determine biochemical parameters by quantitative FRET assays.Acta Pharmacol Sin. 2015 Dec;36(12):1408-15. doi: 10.1038/aps.2015.82. Epub 2015 Nov 16. Acta Pharmacol Sin. 2015. PMID: 26567729 Free PMC article. Review.
Cited by
-
SUMO downregulates GLP-1-stimulated cAMP generation and insulin secretion.Am J Physiol Endocrinol Metab. 2012 Mar 15;302(6):E714-23. doi: 10.1152/ajpendo.00486.2011. Epub 2012 Jan 10. Am J Physiol Endocrinol Metab. 2012. PMID: 22234371 Free PMC article.
-
Fluorescence strategies for high-throughput quantification of protein interactions.Nucleic Acids Res. 2012 Mar;40(5):e33. doi: 10.1093/nar/gkr1045. Epub 2011 Nov 24. Nucleic Acids Res. 2012. PMID: 22121211 Free PMC article.
-
An ISG15-Based High-Throughput Screening Assay for Identification and Characterization of SARS-CoV-2 Inhibitors Targeting Papain-like Protease.Viruses. 2024 Aug 1;16(8):1239. doi: 10.3390/v16081239. Viruses. 2024. PMID: 39205213 Free PMC article.
-
Protein-Protein Affinity Determination by Quantitative FRET Quenching.Sci Rep. 2019 Feb 14;9(1):2050. doi: 10.1038/s41598-018-35535-9. Sci Rep. 2019. PMID: 30765720 Free PMC article.
-
Internal calibration Förster resonance energy transfer assay: a real-time approach for determining protease kinetics.Sensors (Basel). 2013 Apr 8;13(4):4553-70. doi: 10.3390/s130404553. Sensors (Basel). 2013. PMID: 23567524 Free PMC article.
References
-
- Alarcon-Vargas D, Ronai Z. SUMO in cancer–wrestlers wanted. Cancer Biol. Ther. 2002;1(3):237–242. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

