Acetaminophen induces apoptosis in rat cortical neurons
- PMID: 21170329
- PMCID: PMC3000821
- DOI: 10.1371/journal.pone.0015360
Acetaminophen induces apoptosis in rat cortical neurons
Abstract
Background: Acetaminophen (AAP) is widely prescribed for treatment of mild pain and fever in western countries. It is generally considered a safe drug and the most frequently reported adverse effect associated with acetaminophen is hepatotoxicity, which generally occurs after acute overdose. During AAP overdose, encephalopathy might develop and contribute to morbidity and mortality. Our hypothesis is that AAP causes direct neuronal toxicity contributing to the general AAP toxicity syndrome.
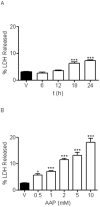
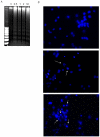
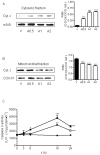
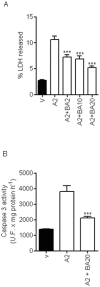
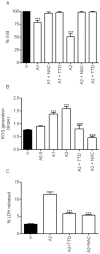
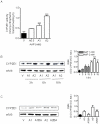
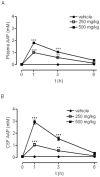
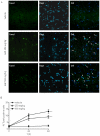
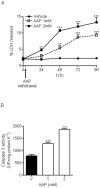
Methodology/principal findings: We report that AAP causes direct toxicity on rat cortical neurons both in vitro and in vivo as measured by LDH release. We have found that AAP causes concentration-dependent neuronal death in vitro at concentrations (1 and 2 mM) that are reached in human plasma during AAP overdose, and that are also reached in the cerebrospinal fluid of rats for 3 hours following i.p injection of AAP doses (250 and 500 mg/kg) that are below those required to induce acute hepatic failure in rats. AAP also increases both neuronal cytochrome P450 isoform CYP2E1 enzymatic activity and protein levels as determined by Western blot, leading to neuronal death through mitochondrial-mediated mechanisms that involve cytochrome c release and caspase 3 activation. In addition, in vivo experiments show that i.p. AAP (250 and 500 mg/kg) injection induces neuronal death in the rat cortex as measured by TUNEL, validating the in vitro data.
Conclusions/significance: The data presented here establish, for the first time, a direct neurotoxic action by AAP both in vivo and in vitro in rats at doses below those required to produce hepatotoxicity and suggest that this neurotoxicity might be involved in the general toxic syndrome observed during patient APP overdose and, possibly, also when AAP doses in the upper dosing schedule are used, especially if other risk factors (moderate drinking, fasting, nutritional impairment) are present.
Conflict of interest statement
Figures
Similar articles
-
Acetaminophen induces apoptosis of C6 glioma cells by activating the c-Jun NH(2)-terminal protein kinase-related cell death pathway.Mol Pharmacol. 2001 Oct;60(4):847-56. Mol Pharmacol. 2001. PMID: 11562448
-
Using acetaminophen's toxicity mechanism to enhance cisplatin efficacy in hepatocarcinoma and hepatoblastoma cell lines.Neoplasia. 2009 Oct;11(10):1003-11. doi: 10.1593/neo.09688. Neoplasia. 2009. PMID: 19794959 Free PMC article.
-
Acetaminophen induces human neuroblastoma cell death through NFKB activation.PLoS One. 2012;7(11):e50160. doi: 10.1371/journal.pone.0050160. Epub 2012 Nov 16. PLoS One. 2012. PMID: 23166834 Free PMC article.
-
Alcohol-mediated increases in acetaminophen hepatotoxicity: role of CYP2E and CYP3A.Biochem Pharmacol. 1998 May 15;55(10):1557-65. doi: 10.1016/s0006-2952(97)00656-4. Biochem Pharmacol. 1998. PMID: 9633991 Review.
-
Acetaminophen.2016 Jan 28. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012–. 2016 Jan 28. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012–. PMID: 31643491 Free Books & Documents. Review.
Cited by
-
Evaluating the Role of Susceptibility Inducing Cofactors and of Acetaminophen in the Etiology of Autism Spectrum Disorder.Life (Basel). 2024 Jul 23;14(8):918. doi: 10.3390/life14080918. Life (Basel). 2024. PMID: 39202661 Free PMC article.
-
Small molecule inhibitors of α-synuclein oligomers identified by targeting early dopamine-mediated motor impairment in C. elegans.Mol Neurodegener. 2021 Nov 12;16(1):77. doi: 10.1186/s13024-021-00497-6. Mol Neurodegener. 2021. PMID: 34772429 Free PMC article.
-
The Role of Vitamin E in Protecting against Oxidative Stress, Inflammation, and the Neurotoxic Effects of Acute Paracetamol in Pregnant Female Rats.Toxics. 2023 Apr 12;11(4):368. doi: 10.3390/toxics11040368. Toxics. 2023. PMID: 37112594 Free PMC article.
-
Prenatal Exposure to Acetaminophen and Risk of ADHD.Pediatrics. 2017 Nov;140(5):e20163840. doi: 10.1542/peds.2016-3840. Pediatrics. 2017. PMID: 29084830 Free PMC article.
-
Prenatal and perinatal analgesic exposure and autism: an ecological link.Environ Health. 2013 May 9;12:41. doi: 10.1186/1476-069X-12-41. Environ Health. 2013. PMID: 23656698 Free PMC article.
References
-
- Botting RM. Mechanism of action of acetaminophen: is there a cyclooxygenase 3. Clin Infect Dis. 2000;31(Suppl 5):S202–S210. - PubMed
-
- Wilcox CM, Cryer B, Triadafilopoulos G. Patterns of use and public perception of over-the-counter pain relievers: focus on nonsteroidal antiinflammatory drugs. J Rheumatol. 2005;32:2218–2224. - PubMed
-
- Kurtovic J, Riordan SM. Paracetamol-induced hepatotoxicity at recommended dosage. J Intern Med. 2003;253:240–243. - PubMed
-
- Bolesta S, Haber SL. Hepatotoxicity associated with chronic acetaminophen administration in patients without risk factors. Ann Pharmacother. 2002;36:331–333. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

