Comparative 3D QSAR study on β(1)-, β(2)-, and β(3)-adrenoceptor agonists
- PMID: 21170122
- PMCID: PMC2988205
- DOI: 10.1007/s00044-009-9257-x
Comparative 3D QSAR study on β(1)-, β(2)-, and β(3)-adrenoceptor agonists
Abstract
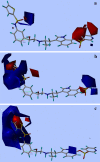
A quantitative structure-activity relationship study of tryptamine-based derivatives of β(1)-, β(2)-, and β(3)-adrenoceptor agonists was conducted using comparative molecular field analysis (CoMFA). Correlation coefficients (cross-validated r(2)) of 0.578, 0.595, and 0.558 were obtained for the three subtypes, respectively, in three different CoMFA models. All three CoMFA models have different steric and electrostatic contributions, implying different requirements inside the binding cavity. The CoMFA coefficient contour plots of the three models and comparisons among these plots provide clues regarding the main chemical features responsible for the biological activity variations and also result in predictions which correlate very well with the observed biological activity. Based on the analysis, a summary regeospecific description of the requirements for improving β-adrenoceptor subtype selectivity is given.
Figures

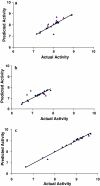
 ) Training set; (
) Training set; ( ) test set
) test set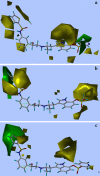
Similar articles
-
Development of 3D-QSAR models for predicting the activities of chemicals to stimulate muscle growth via β2-adrenoceptor.Toxicol In Vitro. 2021 Dec;77:105251. doi: 10.1016/j.tiv.2021.105251. Epub 2021 Sep 30. Toxicol In Vitro. 2021. PMID: 34601065
-
3D-QSAR of human immunodeficiency virus (I) protease inhibitors. III. Interpretation of CoMFA results.Drug Des Discov. 1994 Jul;12(1):29-51. Drug Des Discov. 1994. PMID: 7578806
-
Flavonoid derivatives as adenosine receptor antagonists: a comparison of the hypothetical receptor binding site based on a comparative molecular field analysis model.J Med Chem. 1998 Jan 1;41(1):46-52. doi: 10.1021/jm970446z. J Med Chem. 1998. PMID: 9438021 Free PMC article.
-
Three-dimensional quantitative structure activity relationship (QSAR) of cytotoxic active 3,5-diaryl-4,5-dihydropyrazole analogs: a comparative molecular field analysis (CoMFA) revisited study.Chem Cent J. 2012 May 30;6(1):50. doi: 10.1186/1752-153X-6-50. Chem Cent J. 2012. PMID: 22647291 Free PMC article.
-
3D-QSAR comparative molecular field analysis on opioid receptor antagonists: pooling data from different studies.J Med Chem. 2005 Mar 10;48(5):1620-9. doi: 10.1021/jm049117e. J Med Chem. 2005. PMID: 15743203
References
-
- Arch JRS, Wilson S. Prospects for beta 3-adrenoceptor agonists in the treatment of obesity and diabetes. Int J Obes Relat Metab Disord. 1996;20:191–199. - PubMed
-
- Biftu T, Feng DD, Liang GB, Kuo H, Qian X, Naylor EM, Colandrea VJ, Candelore MR, Cascieri MA, Colwell LF, Jr, Forrest MJ, Hom GJ, MacIntyre DE, Stearns RA, Strader CD, Wyvratt MJ, Fisher MH, Weber AE. Synthesis and SAR of benzyl and phenoxymethylene oxadiazole benzenesulfonamides as selective beta3 adrenergic receptor agonist antiobesity agents. Bioorg Med Chem Lett. 2000;10:1431–1434. doi: 10.1016/S0960-894X(00)00268-7. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Chemical Information
