The filamins: organizers of cell structure and function
- PMID: 21169733
- PMCID: PMC3084982
- DOI: 10.4161/cam.5.2.14401
The filamins: organizers of cell structure and function
Abstract
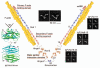
Filamin A (FLNa), the first non-muscle actin filament cross-linking protein, was identified in 1975. Thirty five years of FLNa research has revealed its structure in great detail, discovered its isoforms (FLNb and c), and identified over 90 binding partners including channels, receptors, intracellular signaling molecules, and even transcription factors. Due to this diversity, mutations in human FLN genes result in a wide range of anomalies with moderate to lethal consequences. This review focuses on the structure and functions of FLNa in cell migration and adhesion.
Figures

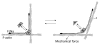

Similar articles
-
Filamin depletion blocks endoplasmic spreading and destabilizes force-bearing adhesions.Mol Biol Cell. 2011 Apr 15;22(8):1263-73. doi: 10.1091/mbc.E10-08-0661. Epub 2011 Feb 16. Mol Biol Cell. 2011. PMID: 21325628 Free PMC article.
-
Filamin A controls matrix metalloproteinase activity and regulates cell invasion in human fibrosarcoma cells.J Cell Sci. 2012 Aug 15;125(Pt 16):3858-69. doi: 10.1242/jcs.104018. Epub 2012 May 17. J Cell Sci. 2012. PMID: 22595522 Free PMC article.
-
Adapter protein SH2B1beta binds filamin A to regulate prolactin-dependent cytoskeletal reorganization and cell motility.Mol Endocrinol. 2011 Jul;25(7):1231-43. doi: 10.1210/me.2011-0056. Epub 2011 May 12. Mol Endocrinol. 2011. PMID: 21566085 Free PMC article.
-
Structural and functional aspects of filamins.Biochim Biophys Acta. 2001 Apr 23;1538(2-3):99-117. doi: 10.1016/s0167-4889(01)00072-6. Biochim Biophys Acta. 2001. PMID: 11336782 Review.
-
New insights into the versatile roles of platelet FlnA.Platelets. 2013;24(1):1-5. doi: 10.3109/09537104.2011.654004. Epub 2012 Feb 28. Platelets. 2013. PMID: 22372530 Free PMC article. Review.
Cited by
-
A mechanism of global shape-dependent recognition and phosphorylation of filamin by protein kinase A.J Biol Chem. 2015 Mar 27;290(13):8527-38. doi: 10.1074/jbc.M114.633446. Epub 2015 Feb 9. J Biol Chem. 2015. PMID: 25666618 Free PMC article.
-
FLN-1/filamin is required to anchor the actomyosin cytoskeleton and for global organization of sub-cellular organelles in a contractile tissue.Cytoskeleton (Hoboken). 2020 Oct;77(10):379-398. doi: 10.1002/cm.21633. Epub 2020 Oct 8. Cytoskeleton (Hoboken). 2020. PMID: 32969593 Free PMC article.
-
Evolutionary Relationships and Divergence of Filamin Gene Family Involved in Development and Stress in Cotton (Gossypium hirsutum L.).Genes (Basel). 2022 Dec 8;13(12):2313. doi: 10.3390/genes13122313. Genes (Basel). 2022. PMID: 36553581 Free PMC article.
-
Spatio-temporal profiling of Filamin A RNA-editing reveals ADAR preferences and high editing levels outside neuronal tissues.RNA Biol. 2013 Oct;10(10):1611-7. doi: 10.4161/rna.26216. Epub 2013 Sep 4. RNA Biol. 2013. PMID: 24025532 Free PMC article.
-
Analysis of molecular mechanism for acceleration of polyembryony using gene functional annotation pipeline in Copidosoma floridanum.BMC Genomics. 2020 Feb 11;21(1):152. doi: 10.1186/s12864-020-6559-3. BMC Genomics. 2020. PMID: 32046635 Free PMC article.
References
-
- Hartwig JH, Stossel TP. Isolation and properties of actin, myosin and a new actinbinding protein in rabbit alveolar macrophages. J Biol Chem. 1975;250:5696–5705. - PubMed
-
- Feng Y, Walsh CA. The many faces of filamin: a versatile molecular scaffold for cell motility and signalling. Nat Cell Biol. 2004;6:1034–1038. - PubMed
-
- Robertson SP, Filamin A. phenotypic diversity. Curr Opin Genet Dev. 2005;15:301–307. - PubMed
-
- Stossel TP, Condeelis J, Cooley L, Hartwig JH, Noegel A, Schleicher M, et al. Filamins as integrators of cell mechanics and signalling. Nat Rev Mol Cell Biol. 2001;2:138–145. - PubMed
-
- Popowicz GM, Schleicher M, Noegel AA, Holak TA. Filamins: promiscuous organizers of the cytoskeleton. Trends Biochem Sci. 2006;31:411–419. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
