FoxO transcription factors promote cardiomyocyte survival upon induction of oxidative stress
- PMID: 21159781
- PMCID: PMC3045002
- DOI: 10.1074/jbc.M110.179242
FoxO transcription factors promote cardiomyocyte survival upon induction of oxidative stress
Abstract
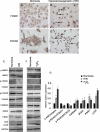
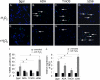
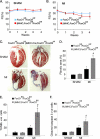
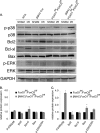
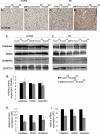
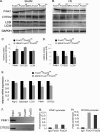
Transcriptional regulatory mechanisms of cardiac oxidative stress resistance are not well defined. FoxO transcription factors are critical mediators of oxidative stress resistance in multiple cell types, but cardioprotective functions have not been reported previously. FoxO function in oxidative stress resistance was investigated in cultured cardiomyocytes and in mice with cardiomyocyte-specific combined deficiency of FoxO1 and FoxO3 subjected to myocardial infarction (MI) or acute ischemia/reperfusion (I/R) injury. Induction of oxidative stress in cardiomyocytes promotes FoxO1 and FoxO3 nuclear localization and target gene activation. Infection of cardiomyocytes with a dominant-negative FoxO1(Δ256) adenovirus results in a significant increase in reactive oxygen species and cell death, whereas increased FoxO1 or FoxO3 expression reduces reactive oxygen species and cell death. Mice generated with combined conditional deletion of FoxO1 and FoxO3 specifically in cardiomyocytes were subjected to I/R or MI. Loss of FoxO1 and FoxO3 in cardiomyocytes results in a significant increase in infarct area with decreased expression of the antiapoptotic molecules, PTEN-induced kinase1 (PINK1) and CBP/P300-interacting transactivator (CITED2). Expressions of the antioxidants catalase and manganese superoxide dismutase-2 (SOD2) and the autophagy-related proteins LC3II and Gabarapl1 also are decreased following I/R compared with controls. Mice with cardiomyocyte-specific FoxO deficiency subjected to MI have reduced cardiac function, increased scar formation, induction of stress-responsive signaling, and increased apoptotic cell death relative to controls. These data support a critical role for FoxOs in promoting cardiomyocyte survival during conditions of oxidative stress through induction of antioxidants and cell survival pathways.
Figures
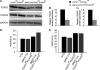
Similar articles
-
FoxO transcription factors promote autophagy in cardiomyocytes.J Biol Chem. 2009 Oct 9;284(41):28319-28331. doi: 10.1074/jbc.M109.024406. Epub 2009 Aug 19. J Biol Chem. 2009. PMID: 19696026 Free PMC article.
-
SIRT6 protects cardiomyocytes against ischemia/reperfusion injury by augmenting FoxO3α-dependent antioxidant defense mechanisms.Basic Res Cardiol. 2016 Mar;111(2):13. doi: 10.1007/s00395-016-0531-z. Epub 2016 Jan 19. Basic Res Cardiol. 2016. PMID: 26786260
-
FOXO1 silence aggravates oxidative stress-promoted apoptosis in cardiomyocytes by reducing autophagy.J Toxicol Sci. 2015;40(5):637-45. doi: 10.2131/jts.40.637. J Toxicol Sci. 2015. PMID: 26354380
-
FoxO1 is crucial for sustaining cardiomyocyte metabolism and cell survival.Cardiovasc Res. 2013 Mar 1;97(3):393-403. doi: 10.1093/cvr/cvs426. Epub 2012 Dec 21. Cardiovasc Res. 2013. PMID: 23263330 Review.
-
Multifaceted functions of the forkhead box transcription factors FoxO1 and FoxO3 in skin.Biochim Biophys Acta Gen Subj. 2017 May;1861(5 Pt A):1057-1064. doi: 10.1016/j.bbagen.2017.02.027. Epub 2017 Feb 27. Biochim Biophys Acta Gen Subj. 2017. PMID: 28249743 Review.
Cited by
-
Activation of EGFR/ERBB2 via pathways involving ERK1/2, P38 MAPK, AKT and FOXO enhances recovery of diabetic hearts from ischemia-reperfusion injury.PLoS One. 2012;7(6):e39066. doi: 10.1371/journal.pone.0039066. Epub 2012 Jun 13. PLoS One. 2012. PMID: 22720029 Free PMC article.
-
AMPK in myocardial infarction and diabetes: the yin/yang effect.Acta Pharm Sin B. 2012 Aug;2(4):368-378. doi: 10.1016/j.apsb.2012.06.001. Epub 2012 Jul 17. Acta Pharm Sin B. 2012. PMID: 32704468 Free PMC article.
-
β-Hydroxybutyrate suppresses inflammasome formation by ameliorating endoplasmic reticulum stress via AMPK activation.Oncotarget. 2016 Oct 11;7(41):66444-66454. doi: 10.18632/oncotarget.12119. Oncotarget. 2016. PMID: 27661104 Free PMC article.
-
Cited2 gene controls pluripotency and cardiomyocyte differentiation of murine embryonic stem cells through Oct4 gene.J Biol Chem. 2012 Aug 17;287(34):29088-100. doi: 10.1074/jbc.M112.378034. Epub 2012 Jul 3. J Biol Chem. 2012. PMID: 22761414 Free PMC article.
-
Adaptive redox response of mesenchymal stromal cells to stimulation with lipopolysaccharide inflammagen: mechanisms of remodeling of tissue barriers in sepsis.Oxid Med Cell Longev. 2013;2013:186795. doi: 10.1155/2013/186795. Epub 2013 Apr 18. Oxid Med Cell Longev. 2013. PMID: 23710283 Free PMC article.
References
-
- Finkel T., Holbrook N. J. (2000) Nature 408, 239–247 - PubMed
-
- Alcendor R. R., Gao S., Zhai P., Zablocki D., Holle E., Yu X., Tian B., Wagner T., Vatner S. F., Sadoshima J. (2007) Circ. Res. 100, 1512–1521 - PubMed
-
- Evans-Anderson H. J., Alfieri C. M., Yutzey K. E. (2008) Circ. Res. 102, 686–694 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

