Single-molecule adhesion forces and attachment lifetimes of myosin-I phosphoinositide interactions
- PMID: 21156133
- PMCID: PMC3000492
- DOI: 10.1016/j.bpj.2010.10.043
Single-molecule adhesion forces and attachment lifetimes of myosin-I phosphoinositide interactions
Abstract
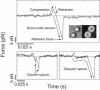
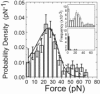
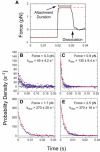
Phosphoinositides regulate the activities and localization of many cytoskeletal proteins involved in crucial biological processes, including membrane-cytoskeleton adhesion. Yet little is known about the mechanics of protein-phosphoinositide interactions, or about the membrane-attachment mechanics of any peripheral membrane proteins. Myosin-Ic (myo1c) is a molecular motor that links membranes to the cytoskeleton via phosphoinositide binding, so it is particularly important to understand the mechanics of its membrane attachment. We used optical tweezers to measure the strength and attachment lifetime of single myo1c molecules as they bind beads coated with a bilayer of 2% phosphatidylinositol 4,5-bisphosphate and 98% phosphatidylcholine. Adhesion forces measured under ramp-load ranged between 5.5 and 16 pN at loading rates between 250 and 1800 pN/s. Dissociation rates increased linearly with constant force (0.3-2.5 pN), with rates exceeding 360 s(-1) at 2.5 pN. Attachment lifetimes calculated from adhesion force measurements were loading-rate-dependent, suggesting nonadiabatic behavior during pulling. The adhesion forces of myo1c with phosphoinositides are greater than the motors stall forces and are within twofold of the force required to extract a lipid molecule from the membrane. However, attachment durations are short-lived, suggesting that phosphoinositides alone do not provide the mechanical stability required to anchor myo1c to membranes during multiple ATPase cycles.
Copyright © 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
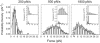
Similar articles
-
Force Generation by Membrane-Associated Myosin-I.Sci Rep. 2016 May 9;6:25524. doi: 10.1038/srep25524. Sci Rep. 2016. PMID: 27156719 Free PMC article.
-
Myosin IC generates power over a range of loads via a new tension-sensing mechanism.Proc Natl Acad Sci U S A. 2012 Sep 11;109(37):E2433-40. doi: 10.1073/pnas.1207811109. Epub 2012 Aug 20. Proc Natl Acad Sci U S A. 2012. PMID: 22908250 Free PMC article.
-
Myo1c binds tightly and specifically to phosphatidylinositol 4,5-bisphosphate and inositol 1,4,5-trisphosphate.Proc Natl Acad Sci U S A. 2006 Feb 28;103(9):3118-23. doi: 10.1073/pnas.0505685103. Epub 2006 Feb 21. Proc Natl Acad Sci U S A. 2006. PMID: 16492791 Free PMC article.
-
Cargo recognition and cargo-mediated regulation of unconventional myosins.Acc Chem Res. 2014 Oct 21;47(10):3061-70. doi: 10.1021/ar500216z. Epub 2014 Sep 17. Acc Chem Res. 2014. PMID: 25230296 Review.
-
Leveraging the membrane - cytoskeleton interface with myosin-1.Trends Cell Biol. 2010 Jul;20(7):418-26. doi: 10.1016/j.tcb.2010.04.004. Epub 2010 May 12. Trends Cell Biol. 2010. PMID: 20471271 Free PMC article. Review.
Cited by
-
Myosin-1A targets to microvilli using multiple membrane binding motifs in the tail homology 1 (TH1) domain.J Biol Chem. 2012 Apr 13;287(16):13104-15. doi: 10.1074/jbc.M111.336313. Epub 2012 Feb 24. J Biol Chem. 2012. PMID: 22367206 Free PMC article.
-
Force Generation by Membrane-Associated Myosin-I.Sci Rep. 2016 May 9;6:25524. doi: 10.1038/srep25524. Sci Rep. 2016. PMID: 27156719 Free PMC article.
-
Distribution of sialic acids on mucins and gels: a defense mechanism.Biophys J. 2012 Jan 4;102(1):176-84. doi: 10.1016/j.bpj.2011.08.058. Epub 2012 Jan 3. Biophys J. 2012. PMID: 22225812 Free PMC article.
-
Molecular to organismal chirality is induced by the conserved myosin 1D.Science. 2018 Nov 23;362(6417):949-952. doi: 10.1126/science.aat8642. Science. 2018. PMID: 30467170 Free PMC article.
-
Adhesion force and attachment lifetime of the KIF16B-PX domain interaction with lipid membranes.Mol Biol Cell. 2017 Nov 7;28(23):3315-3322. doi: 10.1091/mbc.E17-05-0324. Epub 2017 Sep 20. Mol Biol Cell. 2017. PMID: 28931594 Free PMC article.
References
-
- Yin H.L., Janmey P.A. Phosphoinositide regulation of the actin cytoskeleton. Annu. Rev. Physiol. 2003;65:761–789. - PubMed
-
- Sheetz M.P., Sable J.E., Döbereiner H.G. Continuous membrane-cytoskeleton adhesion requires continuous accommodation to lipid and cytoskeleton dynamics. Annu. Rev. Biophys. Biomol. Struct. 2006;35:417–434. - PubMed
-
- Mashanov G.I., Tacon D., Molloy J.E. The spatial and temporal dynamics of pleckstrin homology domain binding at the plasma membrane measured by imaging single molecules in live mouse myoblasts. J. Biol. Chem. 2004;279:15274–15280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

