A new approach for structure analysis of two-dimensional membrane protein crystals using X-ray powder diffraction data
- PMID: 21154412
- PMCID: PMC3048430
- DOI: 10.1002/pro.572
A new approach for structure analysis of two-dimensional membrane protein crystals using X-ray powder diffraction data
Abstract
The application of powder diffraction methods to problems in structural biology is generally regarded as intractable because of the large number of unresolved, overlapping X-ray reflections. Here, we use information about unit cell lattice parameters, space group transformations, and chemical composition as a priori information in a bootstrap process that resolves the ambiguities associated with overlapping reflections. The measured ratios of reflections that can be resolved experimentally are used to refine the position, the shape, and the orientation of low-resolution molecular structures within the unit cell, in leading to the resolution of the overlapping reflections. The molecular model is then made progressively more sophisticated as additional diffraction information is included in the analysis. We apply our method to the recovery of the structure of the bacteriorhodopsin molecule (bR) to a resolution of 7 Å using experimental data obtained from two-dimensional purple membrane crystals. The approach can be used to determine the structure factors directly or to provide reliable low-resolution phase information that can be refined further by the conventional methods of protein crystallography.
Copyright © 2011 The Protein Society.
Figures
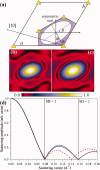
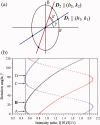
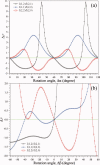
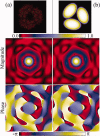
 , where Aφ(S) is the magnitude and ϕφ(S) is the phase of φ(S). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
, where Aφ(S) is the magnitude and ϕφ(S) is the phase of φ(S). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]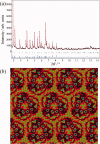
Similar articles
-
Bicelle crystallization: a new method for crystallizing membrane proteins yields a monomeric bacteriorhodopsin structure.J Mol Biol. 2002 Feb 8;316(1):1-6. doi: 10.1006/jmbi.2001.5295. J Mol Biol. 2002. PMID: 11829498
-
Detergent-free membrane protein crystallization.FEBS Lett. 1999 Aug 27;457(2):205-8. doi: 10.1016/s0014-5793(99)01014-5. FEBS Lett. 1999. PMID: 10471779
-
Assessment of the Stoichiometry of Multicomponent Crystals Using Only X-ray Powder Diffraction Data.Mol Pharm. 2015 Jun 1;12(6):2061-7. doi: 10.1021/mp5008458. Epub 2015 Apr 28. Mol Pharm. 2015. PMID: 25872584
-
It's not just a phase: crystallization and X-ray structure determination of bacteriorhodopsin in lipidic cubic phases.Structure. 1998 Jan 15;6(1):5-10. doi: 10.1016/s0969-2126(98)00002-1. Structure. 1998. PMID: 9493262 Review.
-
Bacteriorhodopsin: Would the real structural intermediates please stand up?Biochim Biophys Acta. 2015 Mar;1850(3):536-53. doi: 10.1016/j.bbagen.2014.05.021. Epub 2014 Jun 8. Biochim Biophys Acta. 2015. PMID: 24918316 Review.
References
-
- Lee T, Seeman P, Rajput A, Farley IJ, Hornykiewicz O. Receptor basis for dopaminergic supersensitivity in Parkinson's disease. Nature. 1978;273:59–61. - PubMed
-
- Seeman P. Dopamine receptors and the dopamine hypothesis of schizophrenia. Synapse. 1987;1:133–152. - PubMed
-
- Emilien G, Maloteaux JM, Geurts M, Hoogenberg K, Cragg S. Dopamine receptors—physiological understanding to therapeutic intervention potential. Pharm Ther. 1999;84:133–156. - PubMed
-
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. - PubMed
-
- Dean B, Bymaster FP, Scarr E. Muscarinic receptors in Schizophrenia. Curr Mol Med. 2003;3:419–426. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

