Staging anti-inflammatory therapy in Alzheimer's disease
- PMID: 21152343
- PMCID: PMC2998033
- DOI: 10.3389/fnagi.2010.00142
Staging anti-inflammatory therapy in Alzheimer's disease
Abstract
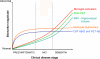
The use of non-steroidal anti-inflammatory drugs (NSAIDs) in Alzheimer's disease (AD) is controversial because conclusions from numerous epidemiological studies reporting delayed onset of AD in NSAID users have not been corroborated in clinical trials. The purpose of this personal view is to revise the case for NSAIDs in AD therapeutics in light of: (i) the last report from the only primary prevention trial in AD, ADAPT, which, although incomplete, points to significant protection in long-term naproxen users, and (ii) the recently proposed dynamic model of AD evolution. The model contends that there is a clinical silent phase in AD that can last up to 20 years, the duration depending on life style habits, genetic factors, or cognitive reserve. The failure of many purported disease-modifying drugs in AD clinical trials is forcing the view that treatments will only be efficacious if administered pre-clinically. Here we will argue that NSAIDs failed in clinical trials because they are disease-modifying drugs, and they should be administered in early stages of the disease. A complete prevention trial in cognitively normal individuals is thus called for. Further, the shift of anti-inflammatory treatment to early stages uncovers a knowledge void about the targets of NSAIDs in asymptomatic individuals. AD researchers have mostly relied on post-mortem analysis of Aβ plaque-laden brains from demented patients or animal models, thus drawing conclusions about AD pathogenesis based on late symptoms. We will discuss evidence in support that defective, not excessive, inflammation underlies AD pathogenesis, that NSAIDs are multifunctional drugs acting on inflammatory and non-inflammatory targets, and that astrocytes and microglia may play differing roles in disease progression, with an emphasis of ApoEε4 as a key, undervalued target of NSAIDs. According to a meta-analysis of epidemiological data, NSAIDs afford an average protection of 58%. If this figure is true, and translated into patient numbers, NSAID treatment may revive as a worth pursuing strategy to significantly reduce the socio-economical burden imposed by AD.
Keywords: ApoE; astrocytes; biomarkers; ibuprofen; microglia; naproxen.
Figures
Similar articles
-
From epidemiology to therapeutic trials with anti-inflammatory drugs in Alzheimer's disease: the role of NSAIDs and cyclooxygenase in beta-amyloidosis and clinical dementia.J Alzheimers Dis. 2002 Oct;4(5):435-45. doi: 10.3233/jad-2002-4510. J Alzheimers Dis. 2002. PMID: 12446975
-
Are NSAIDs useful to treat Alzheimer's disease or mild cognitive impairment?Front Aging Neurosci. 2010 May 21;2:19. doi: 10.3389/fnagi.2010.00019. eCollection 2010. Front Aging Neurosci. 2010. PMID: 20725517 Free PMC article.
-
NSAID and antioxidant prevention of Alzheimer's disease: lessons from in vitro and animal models.Ann N Y Acad Sci. 2004 Dec;1035:68-84. doi: 10.1196/annals.1332.005. Ann N Y Acad Sci. 2004. PMID: 15681801 Review.
-
Role of APOE and Age at Enrollment in the Alzheimer's Disease Anti-Inflammatory Prevention Trial (ADAPT).Dement Geriatr Cogn Dis Extra. 2012 Jan;2(1):304-11. doi: 10.1159/000341783. Epub 2012 Aug 16. Dement Geriatr Cogn Dis Extra. 2012. PMID: 22962554 Free PMC article.
-
Non-steroidal anti-inflammatory drugs (NSAIDs) and other anti-inflammatory agents in the treatment of neurodegenerative disease.Curr Alzheimer Res. 2005 Jul;2(3):355-65. doi: 10.2174/1567205054367883. Curr Alzheimer Res. 2005. PMID: 15974901 Review.
Cited by
-
Glutamate and GABA in Microglia-Neuron Cross-Talk in Alzheimer's Disease.Int J Mol Sci. 2021 Oct 28;22(21):11677. doi: 10.3390/ijms222111677. Int J Mol Sci. 2021. PMID: 34769106 Free PMC article. Review.
-
Perspectives in molecular imaging using staging biomarkers and immunotherapies in Alzheimer's disease.ScientificWorldJournal. 2013;2013:589308. doi: 10.1155/2013/589308. Epub 2013 Feb 5. ScientificWorldJournal. 2013. PMID: 23476143 Free PMC article. Review.
-
Potentiating the Benefits of Melatonin through Chemical Functionalization: Possible Impact on Multifactorial Neurodegenerative Disorders.Int J Mol Sci. 2021 Oct 27;22(21):11584. doi: 10.3390/ijms222111584. Int J Mol Sci. 2021. PMID: 34769013 Free PMC article. Review.
-
Nitazoxanide, an anti-parasitic drug, efficiently ameliorates learning and memory impairments in AD model mice.Acta Pharmacol Sin. 2019 Oct;40(10):1279-1291. doi: 10.1038/s41401-019-0220-1. Epub 2019 Apr 18. Acta Pharmacol Sin. 2019. PMID: 31000769 Free PMC article.
-
Exendin-4 protected against cognitive dysfunction in hyperglycemic mice receiving an intrahippocampal lipopolysaccharide injection.PLoS One. 2012;7(7):e39656. doi: 10.1371/journal.pone.0039656. Epub 2012 Jul 23. PLoS One. 2012. PMID: 22844396 Free PMC article.
References
-
- Aisen P. (2010). Treating before symptoms-ADCS invites ideas for clinical trials in very early AD. Alzforum http://www.alzforum.org/res/for/journal/detail.asp?liveID=180
-
- Arends Y. M., Duyckaerts C., Rozemuller J. M., Eikelenboom P., Hauw J. J. (2000). Microglia, amyloid and dementia in alzheimer disease. A correlative study. Neurobiol. Aging 21, 39–4710.1016/S0197-4580(00)00094-410.1016/S0197-4580(00)00094-410.1111/j.1460-9568.2007.05652.x - DOI - DOI - DOI - PubMed
-
- Butovsky O., Kunis G., Koronyo-Hamaoui M., Schwartz M. (2007). Selective ablation of bone marrow-derived dendritic cells increases amyloid plaques in a mouse Alzheimer's disease model. Eur. J. Neurosci. 26, 413–41610.1056/NEJMoa080943710.1111/j.1460-9568.2007.05652.x10.1016/j.tips.2009.01.002 - DOI - DOI - DOI - PubMed
-
- Caselli R. J., Dueck A. C., Osborne D., Sabbagh M. N., Connor D. J., Ahern G. L., Baxter L. C., Rapcsak S. Z., Shi J., Woodruff B. K., Locke D. E., Snyder C. H., Alexander G. E., Rademakers R., Reiman E. M. (2009). Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N. Engl. J. Med. 361, 255–26310.1016/j.nbd.2010.01.01910.1056/NEJMoa0809437 - DOI - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

