Topology of the C-terminal tail of HIV-1 gp41: differential exposure of the Kennedy epitope on cell and viral membranes
- PMID: 21151874
- PMCID: PMC2998427
- DOI: 10.1371/journal.pone.0015261
Topology of the C-terminal tail of HIV-1 gp41: differential exposure of the Kennedy epitope on cell and viral membranes
Abstract
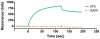
The C-terminal tail (CTT) of the HIV-1 gp41 envelope (Env) protein is increasingly recognized as an important determinant of Env structure and functional properties, including fusogenicity and antigenicity. While the CTT has been commonly referred to as the "intracytoplasmic domain" based on the assumption of an exclusive localization inside the membrane lipid bilayer, early antigenicity studies and recent biochemical analyses have produced a credible case for surface exposure of specific CTT sequences, including the classical "Kennedy epitope" (KE) of gp41, leading to an alternative model of gp41 topology with multiple membrane-spanning domains. The current study was designed to test these conflicting models of CTT topology by characterizing the exposure of native CTT sequences and substituted VSV-G epitope tags in cell- and virion-associated Env to reference monoclonal antibodies (MAbs). Surface staining and FACS analysis of intact, Env-expressing cells demonstrated that the KE is accessible to binding by MAbs directed to both an inserted VSV-G epitope tag and the native KE sequence. Importantly, the VSV-G tag was only reactive when inserted into the KE; no reactivity was observed in cells expressing Env with the VSV-G tag inserted into the LLP2 domain. In contrast to cell-surface expressed Env, no binding of KE-directed MAbs was observed to Env on the surface of intact virions using either immune precipitation or surface plasmon resonance spectroscopy. These data indicate apparently distinct CTT topologies for virion- and cell-associated Env species and add to the case for a reconsideration of CTT topology that is more complex than currently envisioned.
Conflict of interest statement
Figures
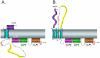




Similar articles
-
Detailed topology mapping reveals substantial exposure of the "cytoplasmic" C-terminal tail (CTT) sequences in HIV-1 Env proteins at the cell surface.PLoS One. 2013 May 27;8(5):e65220. doi: 10.1371/journal.pone.0065220. Print 2013. PLoS One. 2013. PMID: 23724133 Free PMC article.
-
C-terminal tail of human immunodeficiency virus gp41: functionally rich and structurally enigmatic.J Gen Virol. 2013 Jan;94(Pt 1):1-19. doi: 10.1099/vir.0.046508-0. Epub 2012 Oct 17. J Gen Virol. 2013. PMID: 23079381 Free PMC article. Review.
-
Design, expression, and immunogenicity of a soluble HIV trimeric envelope fragment adopting a prefusion gp41 configuration.J Biol Chem. 2005 Jun 17;280(24):23138-46. doi: 10.1074/jbc.M414515200. Epub 2005 Apr 15. J Biol Chem. 2005. PMID: 15833740
-
Unique functional properties of conserved arginine residues in the lentivirus lytic peptide domains of the C-terminal tail of HIV-1 gp41.J Biol Chem. 2014 Mar 14;289(11):7630-40. doi: 10.1074/jbc.M113.529339. Epub 2014 Feb 4. J Biol Chem. 2014. PMID: 24497632 Free PMC article.
-
The frantic play of the concealed HIV envelope cytoplasmic tail.Retrovirology. 2013 May 24;10:54. doi: 10.1186/1742-4690-10-54. Retrovirology. 2013. PMID: 23705972 Free PMC article. Review.
Cited by
-
Cell surface ectodomain integrity of a subset of functional HIV-1 envelopes is dependent on a conserved hydrophilic domain containing region in their C-terminal tail.Retrovirology. 2018 Jul 20;15(1):50. doi: 10.1186/s12977-018-0431-4. Retrovirology. 2018. PMID: 30029604 Free PMC article.
-
Impact of HIV-1 backbone on neutralization sensitivity: neutralization profiles of heterologous envelope glycoproteins expressed in native subtype C and CRF01_AE backbone.PLoS One. 2013 Nov 29;8(11):e76104. doi: 10.1371/journal.pone.0076104. eCollection 2013. PLoS One. 2013. PMID: 24312165 Free PMC article.
-
Highly conserved structural properties of the C-terminal tail of HIV-1 gp41 protein despite substantial sequence variation among diverse clades: implications for functions in viral replication.J Biol Chem. 2011 Aug 5;286(31):27156-66. doi: 10.1074/jbc.M111.258855. Epub 2011 Jun 9. J Biol Chem. 2011. PMID: 21659530 Free PMC article.
-
Kennedy Epitope (KE)-dependent Retrograde Transport of Efficiently Cleaved HIV-1 Envelopes (Envs) and its Effect on Env Cell Surface Expression and Viral Particle Formation.Protein J. 2024 Apr;43(2):375-386. doi: 10.1007/s10930-023-10161-1. Epub 2023 Oct 4. Protein J. 2024. PMID: 37794304
-
Efficiently cleaved HIV-1 envelopes: can they be important for vaccine immunogen development?Ther Adv Vaccines Immunother. 2020 Oct 8;8:2515135520957763. doi: 10.1177/2515135520957763. eCollection 2020. Ther Adv Vaccines Immunother. 2020. PMID: 33103053 Free PMC article. Review.
References
-
- Evans DT, Desrosiers RC. Immune evasion strategies of the primate lentiviruses. Immunol Rev. 2001;183:141–158. - PubMed
-
- Luciw PA. Human immunodeficiency viruses and their replication. In: Fields BN, Knipe DM, Howley PM, editors. Fields' Virology. Third ed. Philadelphia: Lippincott-Raven Publishers; 2002. pp. 1881–1952.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

