High-scatter T cells: a reliable biomarker for malignant T cells in cutaneous T-cell lymphoma
- PMID: 21148332
- PMCID: PMC3056643
- DOI: 10.1182/blood-2010-05-287664
High-scatter T cells: a reliable biomarker for malignant T cells in cutaneous T-cell lymphoma
Abstract
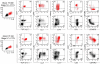
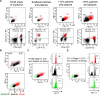
In early-stage cutaneous T-cell lymphoma (CTCL), malignant T cells are confined to skin and are difficult to isolate and discriminate from benign reactive cells. We found that T cells from CTCL skin lesions contained a population of large, high-scatter, activated skin homing T cells not observed in other inflammatory skin diseases. High-scatter T (T(HS)) cells were CD4(+) in CD4(+) mycosis fungoides (MF), CD8(+) in CD8(+) MF, and contained only clonal T cells in patients with identifiable malignant Vβ clones. T(HS) cells were present in the blood of patients with leukemic CTCL, absent in patients without blood involvement, and contained only clonal malignant T cells. The presence of clonal T(HS) cells correlated with skin disease in patients followed longitudinally. Clonal T(HS) cells underwent apoptosis in patients clearing on extracorporeal photopheresis but persisted in nonresponsive patients. Benign clonal T-cell proliferations mapped to the normal low-scatter T-cell population. Thus, the malignant T cells in both MF and leukemic CTCL can be conclusively identified by a unique scatter profile. This observation will allow selective study of malignant T cells, can be used to discriminate patients with MF from patients with other inflammatory skin diseases, to detect peripheral blood involvement, and to monitor responses to therapy.
Figures

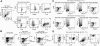




Similar articles
-
Localization of clonal T cells to the epidermis in cutaneous T-cell lymphoma.J Am Acad Dermatol. 1994 Nov;31(5 Pt 1):717-23. doi: 10.1016/s0190-9622(94)70231-4. J Am Acad Dermatol. 1994. PMID: 7929915
-
Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part I. Diagnosis: clinical and histopathologic features and new molecular and biologic markers.J Am Acad Dermatol. 2014 Feb;70(2):205.e1-16; quiz 221-2. doi: 10.1016/j.jaad.2013.07.049. J Am Acad Dermatol. 2014. PMID: 24438969 Review.
-
Normal and cancer fibroblasts differentially regulate TWIST1, TOX and cytokine gene expression in cutaneous T-cell lymphoma.BMC Cancer. 2021 May 3;21(1):492. doi: 10.1186/s12885-021-08142-7. BMC Cancer. 2021. PMID: 33941102 Free PMC article.
-
The relevance of peripheral blood T-helper 1 and 2 cytokine pattern in the evaluation of patients with mycosis fungoides and Sézary syndrome.Br J Dermatol. 2003 Apr;148(4):709-18. doi: 10.1046/j.1365-2133.2003.05224.x. Br J Dermatol. 2003. PMID: 12752128
-
Cutaneous T cell lymphoma: the helping hand of dendritic cells.Ann N Y Acad Sci. 2001 Sep;941:1-11. Ann N Y Acad Sci. 2001. PMID: 11594563 Review.
Cited by
-
Large-cell transformation is an independent poor prognostic factor in Sézary syndrome: analysis of 117 cases.Br J Dermatol. 2022 Nov;187(5):815-817. doi: 10.1111/bjd.21738. Epub 2022 Aug 2. Br J Dermatol. 2022. PMID: 35791764 Free PMC article. No abstract available.
-
The role of cytokine signaling in the pathogenesis of cutaneous T-cell lymphoma.Cancer Biol Ther. 2011 Dec 15;12(12):1019-22. doi: 10.4161/cbt.12.12.18144. Epub 2011 Dec 15. Cancer Biol Ther. 2011. PMID: 22236880 Free PMC article. Review.
-
Sézary Syndrome and Atopic Dermatitis: Comparison of Immunological Aspects and Targets.Biomed Res Int. 2016;2016:9717530. doi: 10.1155/2016/9717530. Epub 2016 May 17. Biomed Res Int. 2016. PMID: 27294147 Free PMC article. Review.
-
[New insights into the pathogenesis and molecular understanding of cutaneous T-cell lymphomas].Dermatologie (Heidelb). 2022 Oct;73(10):765-771. doi: 10.1007/s00105-022-05047-9. Epub 2022 Aug 12. Dermatologie (Heidelb). 2022. PMID: 35960311 Review. German.
-
CD164 and FCRL3 are highly expressed on CD4+CD26- T cells in Sézary syndrome patients.J Invest Dermatol. 2014 Jan;134(1):229-236. doi: 10.1038/jid.2013.279. Epub 2013 Jun 21. J Invest Dermatol. 2014. PMID: 23792457 Free PMC article.
References
-
- Kim YH, Liu HL, Mraz-Gernhard S, Varghese A, Hoppe RT. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139(7):857–866. - PubMed
-
- Fivenson DP, Hanson CA, Nickoloff BJ. Localization of clonal T cells to the epidermis in cutaneous T-cell lymphoma. J Am Acad Dermatol. 1994;31(5 Pt 1):717–723. - PubMed
-
- Ferenczi K, Fuhlbrigge RC, Pinkus J, Pinkus GS, Kupper TS. Increased CCR4 expression incutaneous T cell lymphoma. J Invest Dermatol. 2002;119(6):1405–1410. - PubMed
-
- Ferenczi K, Yawalkar N, Jones D, Kupper TS. Monitoring the decrease of circulating malignant T cells in cutaneous T-cell lymphoma during photopheresis and interferon therapy. Arch Dermatol. 2003;139(7):909–913. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

