Negative regulation of IRF7 activation by activating transcription factor 4 suggests a cross-regulation between the IFN responses and the cellular integrated stress responses
- PMID: 21148039
- PMCID: PMC3057134
- DOI: 10.4049/jimmunol.1002240
Negative regulation of IRF7 activation by activating transcription factor 4 suggests a cross-regulation between the IFN responses and the cellular integrated stress responses
Abstract
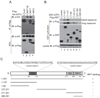
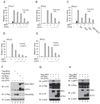
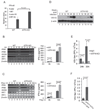
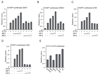
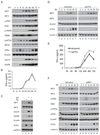
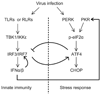
Cells react to viral infection by exhibiting IFN-based innate immune responses and integrated stress responses, but little is known about the interrelationships between the two. In this study, we report a linkage between these two host-protective cellular mechanisms. We found that IFN regulatory factor (IRF)7, the master regulator of type I IFN gene expression, interacts with activating transcription factor (ATF)4, a key component of the integrated stress responses whose translation is induced by viral infection and various stresses. We have demonstrated that IRF7 upregulates ATF4 activity and expression, whereas ATF4 in return inhibits IRF7 activation, suggesting a cross-regulation between the IFN response and the cellular integrated stress response that controls host innate immune defense against viral infection.
Conflict of interest statement
DISCLOSURES
The authors have no financial conflicts of interest.
Figures

Similar articles
-
Tripartite motif-containing protein 28 is a small ubiquitin-related modifier E3 ligase and negative regulator of IFN regulatory factor 7.J Immunol. 2011 Nov 1;187(9):4754-63. doi: 10.4049/jimmunol.1101704. Epub 2011 Sep 21. J Immunol. 2011. PMID: 21940674 Free PMC article.
-
Guanylate binding protein 4 negatively regulates virus-induced type I IFN and antiviral response by targeting IFN regulatory factor 7.J Immunol. 2011 Dec 15;187(12):6456-62. doi: 10.4049/jimmunol.1003691. Epub 2011 Nov 16. J Immunol. 2011. PMID: 22095711
-
Virus-activated interferon regulatory factor 7 upregulates expression of the interferon-regulated BST2 gene independently of interferon signaling.J Virol. 2012 Apr;86(7):3513-27. doi: 10.1128/JVI.06971-11. Epub 2012 Feb 1. J Virol. 2012. PMID: 22301143 Free PMC article.
-
Enhancement and diversification of IFN induction by IRF-7-mediated positive feedback.J Interferon Cytokine Res. 2002 Jan;22(1):87-93. doi: 10.1089/107999002753452692. J Interferon Cytokine Res. 2002. PMID: 11846979 Review.
-
Induction and regulation of IFNs during viral infections.J Interferon Cytokine Res. 2004 Aug;24(8):439-54. doi: 10.1089/1079990041689665. J Interferon Cytokine Res. 2004. PMID: 15320958 Review.
Cited by
-
Tripartite motif-containing protein 28 is a small ubiquitin-related modifier E3 ligase and negative regulator of IFN regulatory factor 7.J Immunol. 2011 Nov 1;187(9):4754-63. doi: 10.4049/jimmunol.1101704. Epub 2011 Sep 21. J Immunol. 2011. PMID: 21940674 Free PMC article.
-
ATF4 Signaling in HIV-1 Infection: Viral Subversion of a Stress Response Transcription Factor.Biology (Basel). 2024 Feb 26;13(3):146. doi: 10.3390/biology13030146. Biology (Basel). 2024. PMID: 38534416 Free PMC article. Review.
-
Innate immune mechanisms in Japanese encephalitis virus infection: effect on transcription of pattern recognition receptors in mouse neuronal cells and brain tissue.Viral Immunol. 2013 Dec;26(6):366-77. doi: 10.1089/vim.2013.0016. Epub 2013 Nov 16. Viral Immunol. 2013. PMID: 24236856 Free PMC article.
-
Host-parasite interaction associated with major mental illness.Mol Psychiatry. 2020 Jan;25(1):194-205. doi: 10.1038/s41380-018-0217-z. Epub 2018 Aug 20. Mol Psychiatry. 2020. PMID: 30127472 Free PMC article.
-
Sex-specific hippocampal 5-hydroxymethylcytosine is disrupted in response to acute stress.Neurobiol Dis. 2016 Dec;96:54-66. doi: 10.1016/j.nbd.2016.08.014. Epub 2016 Aug 26. Neurobiol Dis. 2016. PMID: 27576189 Free PMC article.
References
-
- Honda K, Takaoka A, Taniguchi T. Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity. 2006;25:349–360. - PubMed
-
- Honda K, Taniguchi T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol. 2006;6:644–658. - PubMed
-
- Hiscott J. Triggering the innate antiviral response through IRF-3 activation. J Biol Chem. 2007;282:15325–15329. - PubMed
-
- Honda K, Yanai H, Takaoka A, Taniguchi T. Regulation of the type I IFN induction: a current view. Int Immunol. 2005;17:1367–1378. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

