Pandemic influenza 1918 H1N1 and 1968 H3N2 DNA vaccines induce cross-reactive immunity in ferrets against infection with viruses drifted for decades
- PMID: 21138536
- PMCID: PMC4941650
- DOI: 10.1111/j.1750-2659.2010.00177.x
Pandemic influenza 1918 H1N1 and 1968 H3N2 DNA vaccines induce cross-reactive immunity in ferrets against infection with viruses drifted for decades
Abstract
Background: Alternative influenza vaccines and vaccine production forms are needed as the conventional protein vaccines do not induce broad cross-reactivity against drifted strains. Furthermore, fast vaccine production is especially important in a pandemic situation, and broader vaccine reactivity would diminish the need for frequent change in the vaccine formulations.
Objective: In this study, we compared the ability of pandemic influenza DNA vaccines to induce immunity against distantly related strains within a subtype with the immunity induced by conventional trivalent protein vaccines against homologous virus challenge.
Methods: Ferrets were immunised by particle-mediated epidermal delivery (gene gun) with DNA vaccines based on the haemagglutinin (HA) and neuraminidase (NA) and/or the matrix (M) and nucleoprotein genes of the 1918 H1N1 Spanish influenza pandemic virus or the 1968 H3N2 Hong Kong influenza pandemic virus. The animals were challenged with contemporary H1N1 or H3N2 viruses.
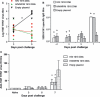
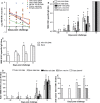
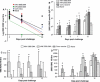
Results: We demonstrated that DNA vaccines encoding proteins of the original 1918 H1N1 pandemic virus induced protective cross-reactive immune responses in ferrets against infection with a 1947 H1N1 virus and a recent 1999 H1N1 virus. Similarly, a DNA vaccine, based on the HA and NA of the 1968 H3N2 pandemic virus, induced cross-reactive immune responses against a recent 2005 H3N2 virus challenge.
Conclusions: DNA vaccines based on pandemic or recent seasonal influenza genes induced cross-reactive immunity against contemporary virus challenge as good as or superior to contemporary conventional trivalent protein vaccines. This suggests a unique ability of influenza DNA to induce cross-protective immunity against both contemporary and long-time drifted viruses.
© 2010 Blackwell Publishing Ltd.
Figures
Similar articles
-
Novel influenza vaccine M2SR protects against drifted H1N1 and H3N2 influenza virus challenge in ferrets with pre-existing immunity.Vaccine. 2018 Aug 9;36(33):5097-5103. doi: 10.1016/j.vaccine.2018.06.053. Epub 2018 Jul 13. Vaccine. 2018. PMID: 30007825 Free PMC article.
-
Rationale for vaccination with trivalent or quadrivalent live attenuated influenza vaccines: Protective vaccine efficacy in the ferret model.PLoS One. 2018 Dec 3;13(12):e0208028. doi: 10.1371/journal.pone.0208028. eCollection 2018. PLoS One. 2018. PMID: 30507951 Free PMC article.
-
Broadly Reactive H2 Hemagglutinin Vaccines Elicit Cross-Reactive Antibodies in Ferrets Preimmune to Seasonal Influenza A Viruses.mSphere. 2021 Mar 10;6(2):e00052-21. doi: 10.1128/mSphere.00052-21. mSphere. 2021. PMID: 33692193 Free PMC article.
-
Recalling the Future: Immunological Memory Toward Unpredictable Influenza Viruses.Front Immunol. 2019 Jul 2;10:1400. doi: 10.3389/fimmu.2019.01400. eCollection 2019. Front Immunol. 2019. PMID: 31312199 Free PMC article. Review.
-
[Tetravaccine--new fundamental approach to prevention of influenza pandemic].Zh Mikrobiol Epidemiol Immunobiol. 2007 Jul-Aug;(4):15-9. Zh Mikrobiol Epidemiol Immunobiol. 2007. PMID: 17882832 Review. Russian.
Cited by
-
Topical and Transdermal Drug Delivery: From Simple Potions to Smart Technologies.Curr Drug Deliv. 2019;16(5):444-460. doi: 10.2174/1567201816666190201143457. Curr Drug Deliv. 2019. PMID: 30714524 Free PMC article. Review.
-
Machine Learning Methods for Predicting Human-Adaptive Influenza A Viruses Based on Viral Nucleotide Compositions.Mol Biol Evol. 2020 Apr 1;37(4):1224-1236. doi: 10.1093/molbev/msz276. Mol Biol Evol. 2020. PMID: 31750915 Free PMC article.
-
A contributing role for anti-neuraminidase antibodies on immunity to pandemic H1N1 2009 influenza A virus.PLoS One. 2011;6(10):e26335. doi: 10.1371/journal.pone.0026335. Epub 2011 Oct 24. PLoS One. 2011. PMID: 22039464 Free PMC article.
-
Protective effect of a polyvalent influenza DNA vaccine in pigs.Vet Immunol Immunopathol. 2018 Jan;195:25-32. doi: 10.1016/j.vetimm.2017.11.007. Epub 2017 Nov 23. Vet Immunol Immunopathol. 2018. PMID: 29249314 Free PMC article.
-
Ferret models of viral pathogenesis.Virology. 2015 May;479-480:259-70. doi: 10.1016/j.virol.2015.03.017. Epub 2015 Mar 26. Virology. 2015. PMID: 25816764 Free PMC article. Review.
References
-
- Demicheli V, Jefferson T, Rivetti D, Deeks J. Prevention and early treatment of influenza in healthy adults. Vaccine 2000; 18(11–12):957–1030. - PubMed
-
- Jefferson TO, Rivetti D, Di Pietrantonj C, Rivetti A, Demicheli V. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev 2007. Apr 18; (2):CD001269. - PubMed
-
- Ulmer JB, Wahren B, Liu MA. Gene‐based vaccines: recent technical and clinical advances. Trends Mol Med 2006; 12(5):216–222. - PubMed
-
- Liu MA, Wahren B, Karlsson Hedestam GB. DNA vaccines: recent developments and future possibilities. Hum Gene Ther 2006; 17(11):1051–1061. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

