Differentiation and regeneration potential of mesenchymal progenitor cells derived from traumatized muscle tissue
- PMID: 21129154
- PMCID: PMC3131486
- DOI: 10.1111/j.1582-4934.2010.01225.x
Differentiation and regeneration potential of mesenchymal progenitor cells derived from traumatized muscle tissue
Abstract
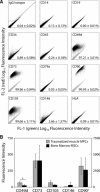
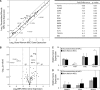
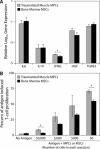
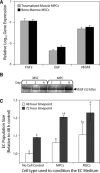
Mesenchymal stem cell (MSC) therapy is a promising approach to promote tissue regeneration by either differentiating the MSCs into the desired cell type or by using their trophic functions to promote endogenous tissue repair. These strategies of regenerative medicine are limited by the availability of MSCs at the point of clinical care. Our laboratory has recently identified multipotent mesenchymal progenitor cells (MPCs) in traumatically injured muscle tissue, and the objective of this study was to compare these cells to a typical population of bone marrow derived MSCs. Our hypothesis was that the MPCs exhibit multilineage differentiation and expression of trophic properties that make functionally them equivalent to bone marrow derived MSCs for tissue regeneration therapies. Quantitative evaluation of their proliferation, metabolic activity, expression of characteristic cell-surface markers and baseline gene expression profile demonstrate substantial similarity between the two cell types. The MPCs were capable of differentiation into osteoblasts, adipocytes and chondrocytes, but they appeared to demonstrate limited lineage commitment compared to the bone marrow derived MSCs. The MPCs also exhibited trophic (i.e. immunoregulatory and pro-angiogenic) properties that were comparable to those of MSCs. These results suggest that the traumatized muscle derived MPCs may not be a direct substitute for bone marrow derived MSCs. However, because of their availability and abundance, particularly following orthopaedic injuries when traumatized muscle is available to harvest autologous cells, MPCs are a promising cell source for regenerative medicine therapies designed to take advantage of their trophic properties.
© 2011 The Authors Journal of Cellular and Molecular Medicine © 2011 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures


Similar articles
-
Mesenchymal progenitor cells derived from traumatized human muscle.J Tissue Eng Regen Med. 2009 Feb;3(2):129-38. doi: 10.1002/term.149. J Tissue Eng Regen Med. 2009. PMID: 19170141 Free PMC article.
-
5-Azacytidine-treated human mesenchymal stem/progenitor cells derived from umbilical cord, cord blood and bone marrow do not generate cardiomyocytes in vitro at high frequencies.Vox Sang. 2008 Aug;95(2):137-48. doi: 10.1111/j.1423-0410.2008.01076.x. Epub 2008 Jun 28. Vox Sang. 2008. PMID: 18557828
-
Combined use of bone marrow-derived mesenchymal stromal cells (BM-MSCs) and platelet rich plasma (PRP) stimulates proliferation and differentiation of myoblasts in vitro: new therapeutic perspectives for skeletal muscle repair/regeneration.Cell Tissue Res. 2018 Jun;372(3):549-570. doi: 10.1007/s00441-018-2792-3. Epub 2018 Feb 5. Cell Tissue Res. 2018. PMID: 29404727
-
Concise Review: Mesenchymal Stem Cells: From Roots to Boost.Stem Cells. 2019 Jul;37(7):855-864. doi: 10.1002/stem.3016. Epub 2019 Apr 30. Stem Cells. 2019. PMID: 30977255 Free PMC article. Review.
-
Musculoskeletal tissue engineering with human umbilical cord mesenchymal stromal cells.Regen Med. 2011 Jan;6(1):95-109. doi: 10.2217/rme.10.98. Regen Med. 2011. PMID: 21175290 Free PMC article. Review.
Cited by
-
Activation of non-myogenic mesenchymal stem cells during the disease progression in dystrophic dystrophin/utrophin knockout mice.Hum Mol Genet. 2015 Jul 1;24(13):3814-29. doi: 10.1093/hmg/ddv125. Epub 2015 Apr 9. Hum Mol Genet. 2015. PMID: 25859011 Free PMC article.
-
Characterization of discrete subpopulations of progenitor cells in traumatic human extremity wounds.PLoS One. 2014 Dec 9;9(12):e114318. doi: 10.1371/journal.pone.0114318. eCollection 2014. PLoS One. 2014. PMID: 25490403 Free PMC article.
-
In Vitro and In Vivo Osteogenesis of Human Orbicularis Oculi Muscle-Derived Stem Cells.Tissue Eng Regen Med. 2018 May 29;15(4):445-452. doi: 10.1007/s13770-018-0122-1. eCollection 2018 Aug. Tissue Eng Regen Med. 2018. PMID: 30603568 Free PMC article.
-
Neurotrophic support by traumatized muscle-derived multipotent progenitor cells: Role of endothelial cells and Vascular Endothelial Growth Factor-A.Stem Cell Res Ther. 2017 Oct 13;8(1):226. doi: 10.1186/s13287-017-0665-4. Stem Cell Res Ther. 2017. PMID: 29029631 Free PMC article.
-
Mesenchymal progenitor cells derived from traumatized muscle enhance neurite growth.J Tissue Eng Regen Med. 2013 Jun;7(6):443-51. doi: 10.1002/term.539. Epub 2012 May 3. J Tissue Eng Regen Med. 2013. PMID: 22552971 Free PMC article.
References
-
- Chen FH, Tuan RS. Adult stem cells for cartilage tissue engineering and regeneration. Curr Rhematol Rev. 2008;4:149–54.
-
- Patterson TE, Kumagai K, Griffith L, et al. Cellular strategies for enhancement of fracture repair. J Bone Joint Surg Am. 2008;90:111–9. - PubMed
-
- Abdallah BM, Kassem M. Human mesenchymal stem cells: from basic biology to clinical applications. Gene Ther. 2007;15:109–16. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

