A metabolic shift induced by a PPAR panagonist markedly reduces the effects of pathogenic mitochondrial tRNA mutations
- PMID: 21129152
- PMCID: PMC3361135
- DOI: 10.1111/j.1582-4934.2010.01223.x
A metabolic shift induced by a PPAR panagonist markedly reduces the effects of pathogenic mitochondrial tRNA mutations
Abstract
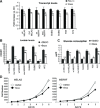
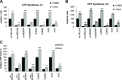
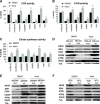
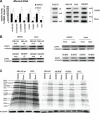
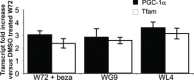
Mutations in mitochondrial DNA-encoded tRNA genes are associated with many human diseases. Activation of peroxisome proliferator-activated receptors (PPARs) by synthetic agonists stimulates oxidative metabolism, induces an increase in mitochondrial mass and partially compensates for oxidative phosphorylation system (OXPHOS) defects caused by single OXPHOS enzyme deficiencies in vitro and in vivo. Here, we analysed whether treatment with the PPAR panagonist bezafibrate in cybrids homoplasmic for different mitochondrial tRNA mutations could ameliorate the OXPHOS defect. We found that bezafibrate treatment increased mitochondrial mass, mitochondrial tRNA steady state levels and enhanced mitochondrial protein synthesis. This improvement resulted in increased OXPHOS activity and finally in enhanced mitochondrial ATP generating capacity. PPAR panagonists are known to increase the expression of PPAR gamma coactivator-1α (PGC-1α), a master regulator of mitochondrial biogenesis. Accordingly, we found that clones of a line harbouring a mutated mitochondrial tRNA gene mutation selected for the ability to grow in a medium selective for OXPHOS function had a 3-fold increase in PGC-1α expression, an increase that was similar to the one observed after bezafibrate treatment. These findings show that increasing mitochondrial mass and thereby boosting residual OXPHOS capacity can be beneficial to an important class of mitochondrial defects reinforcing the potential therapeutic use of approaches stimulating mitochondrial proliferation for mitochondrial disorders.
© 2011 The Authors Journal of Cellular and Molecular Medicine © 2011 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd No claim to original US government works.
Figures
Similar articles
-
Mitochondrial biogenesis: pharmacological approaches.Curr Pharm Des. 2014;20(35):5507-9. doi: 10.2174/138161282035140911142118. Curr Pharm Des. 2014. PMID: 24606795
-
Turn up the power - pharmacological activation of mitochondrial biogenesis in mouse models.Br J Pharmacol. 2014 Apr;171(8):1818-36. doi: 10.1111/bph.12413. Br J Pharmacol. 2014. PMID: 24102298 Free PMC article. Review.
-
Activation of the PPAR/PGC-1alpha pathway prevents a bioenergetic deficit and effectively improves a mitochondrial myopathy phenotype.Cell Metab. 2008 Sep;8(3):249-56. doi: 10.1016/j.cmet.2008.07.006. Cell Metab. 2008. Retraction in: Cell Metab. 2016 Dec 13;24(6):889. doi: 10.1016/j.cmet.2016.11.006. PMID: 18762025 Free PMC article. Retracted.
-
Bezafibrate activation of PPAR drives disturbances in mitochondrial redox bioenergetics and decreases the viability of cells from patients with VLCAD deficiency.Biochim Biophys Acta Mol Basis Dis. 2021 Jun 1;1867(6):166100. doi: 10.1016/j.bbadis.2021.166100. Epub 2021 Feb 5. Biochim Biophys Acta Mol Basis Dis. 2021. PMID: 33549744
-
Dual and pan-peroxisome proliferator-activated receptors (PPAR) co-agonism: the bezafibrate lessons.Cardiovasc Diabetol. 2005 Sep 16;4:14. doi: 10.1186/1475-2840-4-14. Cardiovasc Diabetol. 2005. PMID: 16168052 Free PMC article. Review.
Cited by
-
Role and treatment of mitochondrial DNA-related mitochondrial dysfunction in sporadic neurodegenerative diseases.Curr Pharm Des. 2011;17(31):3356-73. doi: 10.2174/138161211798072535. Curr Pharm Des. 2011. PMID: 21902672 Free PMC article. Review.
-
Botanical formulation HX110B ameliorates PPE-induced emphysema in mice via regulation of PPAR/RXR signaling pathway.PLoS One. 2024 Jul 25;19(7):e0305911. doi: 10.1371/journal.pone.0305911. eCollection 2024. PLoS One. 2024. PMID: 39052574 Free PMC article.
-
Mitochondrial Genetic Disorders: Cell Signaling and Pharmacological Therapies.Cells. 2019 Mar 28;8(4):289. doi: 10.3390/cells8040289. Cells. 2019. PMID: 30925787 Free PMC article. Review.
-
Mitochondrial biogenesis: a therapeutic target for neurodevelopmental disorders and neurodegenerative diseases.Curr Pharm Des. 2014;20(35):5574-93. doi: 10.2174/1381612820666140305224906. Curr Pharm Des. 2014. PMID: 24606804 Free PMC article. Review.
-
Pharmacologic activation of mitochondrial biogenesis exerts widespread beneficial effects in a transgenic mouse model of Huntington's disease.Hum Mol Genet. 2012 Mar 1;21(5):1124-37. doi: 10.1093/hmg/ddr541. Epub 2011 Nov 17. Hum Mol Genet. 2012. PMID: 22095692 Free PMC article.
References
-
- DiMauro S, Schon EA. Mitochondrial disorders in the nervous system. Annu Rev Neurosci. 2008;31:91–123. - PubMed
-
- Perez-Martinez X, Funes S, Camacho-Villasana Y, et al. Protein synthesis and assembly in mitochondrial disorders. Curr Top Med Chem. 2008;8:1335–50. - PubMed
-
- Sasarman F, Antonicka H, Shoubridge EA. The A3243G tRNALeu(UUR) MELAS mutation causes amino acid misincorporation and a combined respiratory chain assembly defect partially suppressed by overexpression of EFTu and EFG2. Hum Mol Genet. 2008;17:3697–707. - PubMed
-
- Bastin J, Aubey F, Rotig A, et al. Activation of peroxisome proliferator-activated receptor pathway stimulates the mitochondrial respiratory chain and can correct deficiencies in patients’ cells lacking its components. J Clin Endocrinol Metab. 2008;93:1433–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

