Adrenergic signaling in human oral keratinocytes and wound repair
- PMID: 21127260
- PMCID: PMC3144102
- DOI: 10.1177/0022034510388034
Adrenergic signaling in human oral keratinocytes and wound repair
Abstract
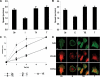
Catecholamines are present in saliva, but their influence on oral epithelium is not understood. Because psychological stress increases salivary catecholamines and impairs oral mucosal wound healing, we sought to determine if epithelial adrenergic signaling could link these two findings. We found that cultured human oral keratinocytes (HOK) express the α(2B)- and β(2)-adrenergic receptors (ARs). Exposure of HOK to either epinephrine or the β-AR agonist, isoproterenol, reduced migratory speed and decreased in vitro scratch wound healing. Incubation with the β-AR antagonist timolol reversed the catecholamine-induced effects, indicating that the observed response is mediated by β-AR. Epinephrine treatment decreased phosphorylation of the mitogen-activated protein kinases (MAPK) ERK1/2 and p38; these decreases were also reversed with timolol. Cultured HOK express enzymes of the epinephrine synthetic pathway, and generate epinephrine. These findings demonstrate that stress-induced elevations of salivary catecholamines signal through MAPK pathways, and result in impaired oral keratinocyte migration required for healing.
Figures

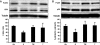

Similar articles
-
Stress-Induced Hormones Cortisol and Epinephrine Impair Wound Epithelization.Adv Wound Care (New Rochelle). 2012 Feb;1(1):29-35. doi: 10.1089/wound.2011.0320. Adv Wound Care (New Rochelle). 2012. PMID: 24527275 Free PMC article. Review.
-
Beta2-adrenergic receptor signaling mediates corneal epithelial wound repair.Invest Ophthalmol Vis Sci. 2008 May;49(5):1857-63. doi: 10.1167/iovs.07-0925. Invest Ophthalmol Vis Sci. 2008. PMID: 18436820 Free PMC article.
-
Stress-mediated increases in systemic and local epinephrine impair skin wound healing: potential new indication for beta blockers.PLoS Med. 2009 Jan 13;6(1):e12. doi: 10.1371/journal.pmed.1000012. PLoS Med. 2009. PMID: 19143471 Free PMC article.
-
Acute wounding alters the beta2-adrenergic signaling and catecholamine synthetic pathways in keratinocytes.J Invest Dermatol. 2014 Aug;134(8):2258-2266. doi: 10.1038/jid.2014.137. Epub 2014 Mar 10. J Invest Dermatol. 2014. PMID: 24614156
-
Beta adrenergic receptors in keratinocytes.Dermatol Clin. 2007 Oct;25(4):643-53, x. doi: 10.1016/j.det.2007.06.012. Dermatol Clin. 2007. PMID: 17903623 Free PMC article. Review.
Cited by
-
Activation of β2-adrenergic receptor by (R,R')-4'-methoxy-1-naphthylfenoterol inhibits proliferation and motility of melanoma cells.Cell Signal. 2015 May;27(5):997-1007. doi: 10.1016/j.cellsig.2015.02.012. Epub 2015 Feb 20. Cell Signal. 2015. PMID: 25703025 Free PMC article.
-
The Ambivalent Role of Skin Microbiota and Adrenaline in Wound Healing and the Interplay between Them.Int J Mol Sci. 2021 May 8;22(9):4996. doi: 10.3390/ijms22094996. Int J Mol Sci. 2021. PMID: 34066786 Free PMC article. Review.
-
Stress-Induced Hormones Cortisol and Epinephrine Impair Wound Epithelization.Adv Wound Care (New Rochelle). 2012 Feb;1(1):29-35. doi: 10.1089/wound.2011.0320. Adv Wound Care (New Rochelle). 2012. PMID: 24527275 Free PMC article. Review.
-
Correlation of Beta-2 Adrenergic Receptor Expression in Tumor-Free Surgical Margin and at the Invasive Front of Oral Squamous Cell Carcinoma.J Oncol. 2016;2016:3531274. doi: 10.1155/2016/3531274. Epub 2016 Mar 2. J Oncol. 2016. PMID: 27042179 Free PMC article.
-
Functional drug-delivery hydrogels for oral and maxillofacial wound healing.Front Bioeng Biotechnol. 2023 Aug 3;11:1241660. doi: 10.3389/fbioe.2023.1241660. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37600316 Free PMC article. Review.
References
-
- Albanidou-Farmaki E, Poulopoulos AK, Epivatianos A, Farmakis K, Karamouzis M, Antoniades D. (2008). Increased anxiety level and high salivary and serum cortisol concentrations in patients with recurrent aphthous stomatitis. Tohoku J Exp Med 214:291-296 - PubMed
-
- Chen J, Hoffman BB, Isseroff RR. (2002). Beta-adrenergic receptor activation inhibits keratinocyte migration via a cyclic adenosine monophosphate-independent mechanism. J Invest Dermatol 119:1261-1268 - PubMed
-
- Fitsialos G, Chassot AA, Turchi L, Dayem MA, LeBrigand K, Moreilhon C, et al. (2007). Transcriptional signature of epidermal keratinocytes subjected to in vitro scratch wounding reveals selective roles for ERK1/2, p38, and phosphatidylinositol 3-kinase signaling pathways. J Biol Chem 282:15090-15102 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

