Intranasal immunization with influenza VLPs incorporating membrane-anchored flagellin induces strong heterosubtypic protection
- PMID: 21124769
- PMCID: PMC2993933
- DOI: 10.1371/journal.pone.0013972
Intranasal immunization with influenza VLPs incorporating membrane-anchored flagellin induces strong heterosubtypic protection
Abstract
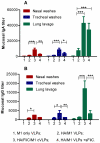
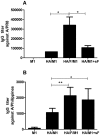
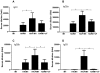
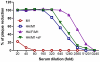
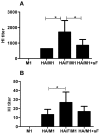
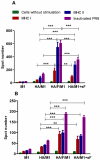
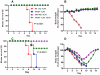
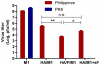
We demonstrated previously that the incorporation of a membrane-anchored form of flagellin into influenza virus-like particles (VLPs) improved the immunogenicity of VLPs significantly, inducing partially protective heterosubtypic immunity by intramuscular immunization. Because the efficacy of mucosal vaccination is highly dependent on an adjuvant, and is particularly effective for preventing mucosal infections such as influenza, we determined whether the membrane-anchored flagellin is an efficient adjuvant for VLP vaccines by a mucosal immunization route. We compared the adjuvant effect of membrane-anchored and soluble flagellins for immunization with influenza A/PR8 (H1N1) VLPs by the intranasal route in a mouse model. The results demonstrate that membrane-anchored flagellin is an effective adjuvant for intranasal (IN) immunization, inducing enhanced systemic and mucosal antibody responses. High cellular responses were also observed as shown by cytokine production in splenocyte cultures when stimulated with viral antigens. All mice immunized with flagellin-containing VLPs survived challenge with a high lethal dose of homologous virus as well as a high dose heterosubtypic virus challenge (40 LD(50) of A/Philippines/82, H3N2). In contrast, no protection was observed with a standard HA/M1 VLP group upon heterosubtypic challenge. Soluble flagellin exhibited a moderate adjuvant effect when co-administered with VLPs by the mucosal route, as indicated by enhanced systemic and mucosal responses and partial heterosubtypic protection. The membrane-anchored form of flagellin incorporated together with antigen into influenza VLPs is effective as an adjuvant by the mucosal route and unlike standard VLPs, immunization with such chimeric VLPs elicits protective immunity to challenge with a distantly related influenza A virus.
Conflict of interest statement
Figures
Similar articles
-
Incorporation of membrane-anchored flagellin into influenza virus-like particles enhances the breadth of immune responses.J Virol. 2008 Dec;82(23):11813-23. doi: 10.1128/JVI.01076-08. Epub 2008 Sep 10. J Virol. 2008. PMID: 18786995 Free PMC article.
-
Intranasal Immunization with Influenza Virus-Like Particles Containing Membrane-Anchored Cholera Toxin B or Ricin Toxin B Enhances Adaptive Immune Responses and Protection against an Antigenically Distinct Virus.Viruses. 2016 Apr 21;8(4):115. doi: 10.3390/v8040115. Viruses. 2016. PMID: 27110810 Free PMC article.
-
Virus-like particles containing the tetrameric ectodomain of influenza matrix protein 2 and flagellin induce heterosubtypic protection in mice.Biomed Res Int. 2013;2013:686549. doi: 10.1155/2013/686549. Epub 2013 Jul 30. Biomed Res Int. 2013. PMID: 23984396 Free PMC article.
-
Potentiating Lung Mucosal Immunity Through Intranasal Vaccination.Front Immunol. 2021 Dec 14;12:808527. doi: 10.3389/fimmu.2021.808527. eCollection 2021. Front Immunol. 2021. PMID: 34970279 Free PMC article. Review.
-
Unravelling influenza correlates of protection: lessons from human A/H1N1 Challenge.mBio. 2024 May 8;15(5):e0006424. doi: 10.1128/mbio.00064-24. Epub 2024 Mar 28. mBio. 2024. PMID: 38546212 Free PMC article. Review.
Cited by
-
Protection against multiple influenza A virus strains induced by candidate recombinant vaccine based on heterologous M2e peptides linked to flagellin.PLoS One. 2015 Mar 23;10(3):e0119520. doi: 10.1371/journal.pone.0119520. eCollection 2015. PLoS One. 2015. PMID: 25799221 Free PMC article.
-
Adjuvantation of Influenza Vaccines to Induce Cross-Protective Immunity.Vaccines (Basel). 2021 Jan 21;9(2):75. doi: 10.3390/vaccines9020075. Vaccines (Basel). 2021. PMID: 33494477 Free PMC article. Review.
-
Virus-like particles as universal influenza vaccines.Expert Rev Vaccines. 2012 Aug;11(8):995-1007. doi: 10.1586/erv.12.70. Expert Rev Vaccines. 2012. PMID: 23002980 Free PMC article. Review.
-
Incorporation of membrane-anchored flagellin or Escherichia coli heat-labile enterotoxin B subunit enhances the immunogenicity of rabies virus-like particles in mice and dogs.Front Microbiol. 2015 Mar 3;6:169. doi: 10.3389/fmicb.2015.00169. eCollection 2015. Front Microbiol. 2015. PMID: 25784906 Free PMC article.
-
Naïve CD4+ T Cell Activation in the Nasal-Associated Lymphoid Tissue following Intranasal Immunization with a Flagellin-Based Subunit Vaccine.Int J Mol Sci. 2022 Dec 8;23(24):15572. doi: 10.3390/ijms232415572. Int J Mol Sci. 2022. PMID: 36555214 Free PMC article.
References
-
- Eriksson K, Holmgren J. Recent advances in mucosal vaccines and adjuvants. Curr Opin Immunol. 2002;14:666–672. - PubMed
-
- Vyas SP, Gupta PN. Implication of nanoparticles/microparticles in mucosal vaccine delivery. Expert Rev Vaccines. 2007;6:401–418. - PubMed
-
- Nugent J, Po AL, Scott EM. Design and delivery of non-parenteral vaccines. J Clin Pharm Ther. 1998;23:257–285. - PubMed
-
- Kuno-Sakai H, Kimura M, Ohta K, Shimojima R, Oh Y, et al. Developments in mucosal influenza virus vaccines. Vaccine. 1994;12:1303–1310. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

