The chromosome passenger complex is required for fidelity of chromosome transmission and cytokinesis in meiosis of mouse oocytes
- PMID: 21123620
- PMCID: PMC2995614
- DOI: 10.1242/jcs.067447
The chromosome passenger complex is required for fidelity of chromosome transmission and cytokinesis in meiosis of mouse oocytes
Abstract
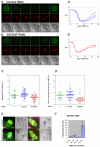
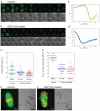
The existence of two forms of the chromosome passenger complex (CPC) in the mammalian oocyte has meant that its role in female meiosis has remained unclear. Here we use loss- and gain-of function approaches to assess the meiotic functions of one of the shared components of these complexes, INCENP, and of the variable kinase subunits, Aurora B or Aurora C. We show that either the depletion of INCENP or the combined inhibition of Aurora kinases B and C activates the anaphase-promoting complex or cyclosome (APC/C) before chromosomes have properly congressed in meiosis I and also prevents cytokinesis and hence extrusion of the first polar body. Overexpression of Aurora C also advances APC/C activation and results in cytokinesis failure in a high proportion of oocytes, indicative of a dominant effect on CPC function. Together, this points to roles for the meiotic CPC in functions similar to the mitotic roles of the complex: correcting chromosome attachment to microtubules, facilitating the spindle-assembly checkpoint (SAC) function and enabling cytokinesis. Surprisingly, overexpression of Aurora B leads to a failure of APC/C activation, stabilization of securin and consequently a failure of chiasmate chromosomes to resolve - a dominant phenotype that is completely suppressed by depletion of INCENP. Taken together with the differential distribution of Aurora proteins B and C on chiasmate chromosomes, this points to differential functions of the two forms of CPC in regulating the separation of homologous chromosomes in meiosis I.
Figures


Similar articles
-
Aurora-C kinase deficiency causes cytokinesis failure in meiosis I and production of large polyploid oocytes in mice.Mol Biol Cell. 2010 Jul 15;21(14):2371-83. doi: 10.1091/mbc.e10-02-0170. Epub 2010 May 19. Mol Biol Cell. 2010. PMID: 20484572 Free PMC article.
-
Switching of INCENP paralogs controls transitions in mitotic chromosomal passenger complex functions.Cell Cycle. 2019 Sep;18(17):2006-2025. doi: 10.1080/15384101.2019.1634954. Epub 2019 Jul 15. Cell Cycle. 2019. PMID: 31306061 Free PMC article.
-
Selective disruption of aurora C kinase reveals distinct functions from aurora B kinase during meiosis in mouse oocytes.PLoS Genet. 2014 Feb 27;10(2):e1004194. doi: 10.1371/journal.pgen.1004194. eCollection 2014 Feb. PLoS Genet. 2014. PMID: 24586209 Free PMC article.
-
Role of chromosomal passenger complex in chromosome segregation and cytokinesis.Cell Struct Funct. 2001 Dec;26(6):653-7. doi: 10.1247/csf.26.653. Cell Struct Funct. 2001. PMID: 11942622 Review.
-
Cell division control by the Chromosomal Passenger Complex.Exp Cell Res. 2012 Jul 15;318(12):1407-20. doi: 10.1016/j.yexcr.2012.03.015. Epub 2012 Mar 24. Exp Cell Res. 2012. PMID: 22472345 Review.
Cited by
-
Mitotic failures in cancer: Aurora B kinase and its potential role in the development of aneuploidy.Pathol Oncol Res. 2012 Oct;18(4):761-9. doi: 10.1007/s12253-012-9534-8. Epub 2012 Jul 29. Pathol Oncol Res. 2012. PMID: 22843098 Review.
-
Aurora kinase A drives MTOC biogenesis but does not trigger resumption of meiosis in mouse oocytes matured in vivo.Biol Reprod. 2012 Oct 11;87(4):85. doi: 10.1095/biolreprod.112.101014. Print 2012 Oct. Biol Reprod. 2012. PMID: 22837479 Free PMC article.
-
Genetic Interactions between the Aurora Kinases Reveal New Requirements for AURKB and AURKC during Oocyte Meiosis.Curr Biol. 2018 Nov 5;28(21):3458-3468.e5. doi: 10.1016/j.cub.2018.08.052. Epub 2018 Oct 25. Curr Biol. 2018. PMID: 30415701 Free PMC article.
-
Possible Role of Aurora-C in Meiosis.Front Oncol. 2015 Aug 13;5:178. doi: 10.3389/fonc.2015.00178. eCollection 2015. Front Oncol. 2015. PMID: 26322271 Free PMC article. Review.
-
Specialize and Divide (Twice): Functions of Three Aurora Kinase Homologs in Mammalian Oocyte Meiotic Maturation.Trends Genet. 2017 May;33(5):349-363. doi: 10.1016/j.tig.2017.03.005. Epub 2017 Mar 27. Trends Genet. 2017. PMID: 28359584 Free PMC article. Review.
References
-
- Bernard M., Sanseau P., Henry C., Couturier A., Prigent C. (1998). Cloning of STK13, a third human protein kinase related to Drosophila aurora and budding yeast Ipl1 that maps on chromosome 19q13.3-ter. Genomics 53, 406-409 - PubMed
-
- Chen H. L., Tang C. J., Chen C. Y., Tang T. K. (2005). Overexpression of an Aurora-C kinase-deficient mutant disrupts the Aurora-B/INCENP complex and induces polyploidy. J. Biomed. Sci. 12, 297-310 - PubMed
-
- Dieterich K., Soto Rifo R., Faure A. K., Hennebicq S., Ben Amar B., Zahi M., Perrin J., Martinez D., Sèle B., Jouk P. S., et al. (2007). Homozygous mutation of AURKC yields large-headed polyploid spermatozoa and causes male infertility. Nat. Genet. 39, 661-665 - PubMed
-
- Dieterich K., Zouari R., Harbuz R., Vialard F., Martinez D., Bellayou H., Prisant N., Zoghmar A., Guichaoua M. R., Koscinski I., et al. (2009). The Aurora Kinase C c.144delC mutation causes meiosis I arrest in men and is frequent in the North African population. Hum. Mol. Genet. 18, 1301-1309 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

