Pharmacological considerations for tenofovir and emtricitabine to prevent HIV infection
- PMID: 21118913
- PMCID: PMC3019086
- DOI: 10.1093/jac/dkq447
Pharmacological considerations for tenofovir and emtricitabine to prevent HIV infection
Abstract
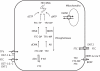
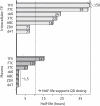
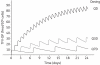
The use of antiretroviral medications in HIV-negative individuals as pre-exposure prophylaxis (PrEP) is a promising approach to prevent HIV infection. Tenofovir disoproxil fumarate (TDF) and emtricitabine exhibit desirable properties for PrEP including: favourable pharmacokinetics that support infrequent dosing; few major drug-drug or drug-food interactions; an excellent clinical safety record; and pre-clinical evidence for efficacy. Several large, randomized, controlled clinical trials are evaluating the safety and efficacy of TDF and emtricitabine for this new indication. A thorough understanding of variability in drug response will help determine future investigations in the field and/or implementation into clinical care. Because tenofovir and emtricitabine are nucleos(t)ide analogues, the HIV prevention and toxicity effects depend on the triphosphate analogue formed intracellularly. This review identifies important cellular pharmacology considerations for tenofovir and emtricitabine, which include drug penetration into relevant tissues and cell types, race/ethnicity/pharmacogenetics, gender, cellular activation state and appropriate episodic or alternative dosing strategies based on pharmacokinetic principles. The current state of knowledge in these areas is summarized and the future utility of intracellular pharmacokinetics/pharmacodynamics for the PrEP field is discussed.
Figures

Similar articles
-
Emtricitabine/tenofovir disoproxil fumarate: a review of its use in HIV-1 pre-exposure prophylaxis.Drugs. 2013 Mar;73(3):279-91. doi: 10.1007/s40265-013-0024-4. Drugs. 2013. PMID: 23444256 Review.
-
Single-agent tenofovir versus combination emtricitabine plus tenofovir for pre-exposure prophylaxis for HIV-1 acquisition: an update of data from a randomised, double-blind, phase 3 trial.Lancet Infect Dis. 2014 Nov;14(11):1055-1064. doi: 10.1016/S1473-3099(14)70937-5. Epub 2014 Oct 7. Lancet Infect Dis. 2014. PMID: 25300863 Free PMC article. Clinical Trial.
-
Randomized, phase 2 evaluation of two single-tablet regimens elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate versus efavirenz/emtricitabine/tenofovir disoproxil fumarate for the initial treatment of HIV infection.AIDS. 2011 Mar 27;25(6):F7-12. doi: 10.1097/QAD.0b013e328345766f. AIDS. 2011. PMID: 21412057 Clinical Trial.
-
Emtricitabine/rilpivirine/tenofovir disoproxil fumarate single-tablet regimen: a guide to its use in HIV-1 infection.Clin Drug Investig. 2012 Oct 1;32(10):715-22. doi: 10.1007/BF03261925. Clin Drug Investig. 2012. PMID: 22921088
-
Oral preexposure anti-HIV prophylaxis for high-risk U.S. populations: current considerations in light of new findings.AIDS Patient Care STDS. 2011 Feb;25(2):63-71. doi: 10.1089/apc.2010.0222. AIDS Patient Care STDS. 2011. PMID: 21284497 Review.
Cited by
-
A Pharmacokinetic/Pharmacodynamic Model to Predict Effective HIV Prophylaxis Dosing Strategies for People Who Inject Drugs.J Pharmacol Exp Ther. 2018 Nov;367(2):245-251. doi: 10.1124/jpet.118.251009. Epub 2018 Aug 27. J Pharmacol Exp Ther. 2018. PMID: 30150483 Free PMC article.
-
Antiviral activity of genital tract secretions after oral or topical tenofovir pre-exposure prophylaxis for HIV-1.J Acquir Immune Defic Syndr. 2014 May 1;66(1):65-73. doi: 10.1097/QAI.0000000000000110. J Acquir Immune Defic Syndr. 2014. PMID: 24457633 Free PMC article.
-
Tenofovir and tenofovir-diphosphate concentrations during pregnancy among HIV-uninfected women using oral preexposure prophylaxis.AIDS. 2018 Aug 24;32(13):1891-1898. doi: 10.1097/QAD.0000000000001922. AIDS. 2018. PMID: 29894385 Free PMC article.
-
Effect of polyphenols against complications of COVID-19: current evidence and potential efficacy.Pharmacol Rep. 2024 Apr;76(2):307-327. doi: 10.1007/s43440-024-00585-6. Epub 2024 Mar 18. Pharmacol Rep. 2024. PMID: 38498260 Review.
-
An intravaginal ring for the sustained delivery of tenofovir disoproxil fumarate.Int J Pharm. 2015 Nov 10;495(1):579-587. doi: 10.1016/j.ijpharm.2015.09.028. Epub 2015 Sep 16. Int J Pharm. 2015. PMID: 26386138 Free PMC article.
References
-
- UNAIDS. 09 AIDS Epidemic Update. November 2009. http://data.unaids.org/pub/Report/2009/2009_epidemic_update_en.pdf. (15 March 2010, date last accessed)
-
- Dolin R. HIV vaccine trial results—an opening for further research. N Engl J Med. 2009;361:2279–80. - PubMed
-
- Cohen MS, Gay C, Kashuba AD, et al. Narrative review: antiretroviral therapy to prevent the sexual transmission of HIV-1. Ann Intern Med. 2007;146:591–601. - PubMed
-
- Garcia-Lerma JG, Paxton L, Kilmarx PH, et al. Oral pre-exposure prophylaxis for HIV prevention. Trends Pharmacol Sci. 2010;31:74–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

