Palmitoylation controls dopamine transporter kinetics, degradation, and protein kinase C-dependent regulation
- PMID: 21118819
- PMCID: PMC3037630
- DOI: 10.1074/jbc.M110.187872
Palmitoylation controls dopamine transporter kinetics, degradation, and protein kinase C-dependent regulation
Abstract
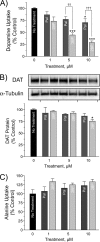
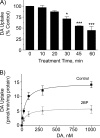
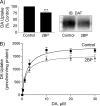
Palmitoylation is a lipid modification that confers diverse functions to target proteins and is a contributing factor for many neuronal diseases. In this study, we demonstrate using [(3)H]palmitic acid labeling and acyl-biotinyl exchange that native and expressed dopamine transporters (DATs) are palmitoylated, and using the palmitoyl acyltransferase inhibitor 2-bromopalmitate (2BP), we identify several associated functions. Treatment of rat striatal synaptosomes with 2BP using lower doses or shorter times caused robust inhibition of transport V(max) that occurred with no losses of DAT protein or changes in DAT surface levels, indicating that acute loss of palmitoylation leads to reduction of transport kinetics. Treatment of synaptosomes or cells with 2BP using higher doses or longer times resulted in DAT protein losses and production of transporter fragments, implicating palmitoylation in regulation of transporter degradation. Site-directed mutagenesis indicated that palmitoylation of rat DAT occurs at Cys-580 at the intracellular end of transmembrane domain 12 and at one or more additional unidentified site(s). Cys-580 mutation also led to production of transporter degradation fragments and to increased phorbol ester-induced down-regulation, further supporting palmitoylation in opposing DAT turnover and in opposing protein kinase C-mediated regulation. These results identify S-palmitoylation as a major regulator of DAT properties that could significantly impact acute and long term dopamine transport capacity.
Figures

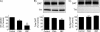


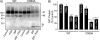
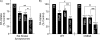
Similar articles
-
Palmitoylation by Multiple DHHC Enzymes Enhances Dopamine Transporter Function and Stability.ACS Chem Neurosci. 2019 Jun 19;10(6):2707-2717. doi: 10.1021/acschemneuro.8b00558. Epub 2019 Apr 19. ACS Chem Neurosci. 2019. PMID: 30965003 Free PMC article.
-
Reciprocal Phosphorylation and Palmitoylation Control Dopamine Transporter Kinetics.J Biol Chem. 2015 Nov 27;290(48):29095-105. doi: 10.1074/jbc.M115.667055. Epub 2015 Sep 30. J Biol Chem. 2015. PMID: 26424792 Free PMC article.
-
Protein kinase C-mediated phosphorylation and functional regulation of dopamine transporters in striatal synaptosomes.J Biol Chem. 1997 Jun 13;272(24):15541-6. doi: 10.1074/jbc.272.24.15541. J Biol Chem. 1997. PMID: 9182590
-
Palmitoylation mechanisms in dopamine transporter regulation.J Chem Neuroanat. 2017 Oct;83-84:3-9. doi: 10.1016/j.jchemneu.2017.01.002. Epub 2017 Jan 20. J Chem Neuroanat. 2017. PMID: 28115272 Free PMC article. Review.
-
Palmitoylation of solute carriers.Biochem Pharmacol. 2023 Sep;215:115695. doi: 10.1016/j.bcp.2023.115695. Epub 2023 Jul 20. Biochem Pharmacol. 2023. PMID: 37481134 Free PMC article. Review.
Cited by
-
Amphetamines, new psychoactive drugs and the monoamine transporter cycle.Trends Pharmacol Sci. 2015 Jan;36(1):41-50. doi: 10.1016/j.tips.2014.11.006. Epub 2014 Dec 23. Trends Pharmacol Sci. 2015. PMID: 25542076 Free PMC article. Review.
-
Attention deficit/hyperactivity disorder-derived coding variation in the dopamine transporter disrupts microdomain targeting and trafficking regulation.J Neurosci. 2012 Apr 18;32(16):5385-97. doi: 10.1523/JNEUROSCI.6033-11.2012. J Neurosci. 2012. PMID: 22514303 Free PMC article.
-
Identification of a Vav2-dependent mechanism for GDNF/Ret control of mesolimbic DAT trafficking.Nat Neurosci. 2015 Aug;18(8):1084-93. doi: 10.1038/nn.4060. Epub 2015 Jul 6. Nat Neurosci. 2015. PMID: 26147533
-
Neogenin interacts with matriptase-2 to facilitate hemojuvelin cleavage.J Biol Chem. 2012 Oct 12;287(42):35104-35117. doi: 10.1074/jbc.M112.363937. Epub 2012 Aug 14. J Biol Chem. 2012. PMID: 22893705 Free PMC article.
-
Increased novelty-induced locomotion, sensitivity to amphetamine, and extracellular dopamine in striatum of Zdhhc15-deficient mice.Transl Psychiatry. 2021 Jan 18;11(1):65. doi: 10.1038/s41398-020-01194-6. Transl Psychiatry. 2021. PMID: 33462194 Free PMC article.
References
-
- Bannon M. J., Sacchetti P., Granneman J. G. (1998) in Psychopharmacology. The Fourth Generation of Progress (Bloom K. ed) on-line edition, Raven Press, Ltd., New York
-
- Giros B., Jaber M., Jones S. R., Wightman R. M., Caron M. G. (1996) Nature 379, 606–612 - PubMed
-
- Miller G. W., Gainetdinov R. R., Levey A. I., Caron M. G. (1999) Trends Pharmacol. Sci. 20, 424–429 - PubMed
-
- Sotnikova T. D., Beaulieu J. M., Gainetdinov R. R., Caron M. G. (2006) CNS Neurol. Disord. Drug Targets 5, 45–56 - PubMed
-
- Sulzer D., Sonders M. S., Poulsen N. W., Galli A. (2005) Prog. Neurobiol. 75, 406–433 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

