Dopamine Release and Uptake Impairments and Behavioral Alterations Observed in Mice that Model Fragile X Mental Retardation Syndrome
- PMID: 21116467
- PMCID: PMC2992329
- DOI: 10.1021/cn100032f
Dopamine Release and Uptake Impairments and Behavioral Alterations Observed in Mice that Model Fragile X Mental Retardation Syndrome
Abstract
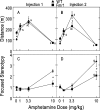
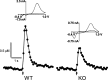
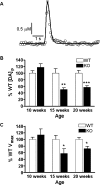
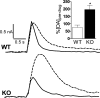
In this study we evaluated the relationship between amphetamine-induced behavioral alterations and dopamine release and uptake characteristics in Fmr1 knockout (Fmr1 KO) mice, which model fragile X syndrome. The behavioral analyses, obtained at millisecond temporal resolution and 2 mm spatial resolution using a force-plate actometer, revealed that Fmr1 KO mice express a lower degree of focused stereotypy compared to wild type (WT) control mice after injection with 10 mg/kg (ip) amphetamine. To identify potentially related neurochemical mechanisms underlying this phenomenon, we measured electrically-evoked dopamine release and uptake using fast-scan cyclic voltammetry at carbon-fiber microelectrodes in striatal brain slices. At 10 weeks of age, dopamine release per pulse, which is dopamine release corrected for differences in uptake, was unchanged. However, at 15 (the age of behavioral testing) and 20 weeks of age, dopamine per pulse and the maximum rate of dopamine uptake was diminished in Fmr1 KO mice compared to WT mice. Dopamine uptake measurements, obtained at different amphetamine concentrations, indicated that dopamine transporters in both genotypes have equal affinities for amphetamine. Moreover, dopamine release measurements from slices treated with quinpirole, a D2-family receptor agonist, rule out enhanced D2 autoreceptor sensitivity as a mechanism of release inhibition. However, dopamine release, uncorrected for uptake and normalized against the corresponding pre-drug release peaks, increased in Fmr1 KO mice, but not in WT mice. Collectively, these data are consistent with a scenario in which a decrease in extracellular dopamine levels in the striatum result in diminished expression of focused stereotypy in Fmr1 KO mice.
Figures


Similar articles
-
Dopamine autoreceptor regulation of release and uptake in mouse brain slices in the absence of D(3) receptors.Neuroscience. 2002;112(1):39-49. doi: 10.1016/s0306-4522(02)00067-2. Neuroscience. 2002. PMID: 12044470
-
Object recognition impairment in Fmr1 knockout mice is reversed by amphetamine: involvement of dopamine in the medial prefrontal cortex.Behav Pharmacol. 2004 Sep;15(5-6):433-42. doi: 10.1097/00008877-200409000-00018. Behav Pharmacol. 2004. PMID: 15343070
-
Dopamine Dysregulation and Altered Responses to Drugs Affecting Dopaminergic Transmission in a New Dopamine Transporter Knockout (DAT-KO) Rat Model.Neuroscience. 2022 May 21;491:43-64. doi: 10.1016/j.neuroscience.2022.03.019. Epub 2022 Mar 21. Neuroscience. 2022. PMID: 35331847
-
Taurine regulation of short term synaptic plasticity in fragile X mice.J Biomed Sci. 2010 Aug 24;17 Suppl 1(Suppl 1):S15. doi: 10.1186/1423-0127-17-S1-S15. J Biomed Sci. 2010. PMID: 20804589 Free PMC article.
-
Motor function and dopamine release measurements in transgenic Huntington's disease model rats.Brain Res. 2012 Apr 23;1450:148-56. doi: 10.1016/j.brainres.2012.02.042. Epub 2012 Feb 24. Brain Res. 2012. PMID: 22418060 Free PMC article.
Cited by
-
ErbB inhibition rescues nigral dopamine neuron hyperactivity and repetitive behaviors in a mouse model of fragile X syndrome.Mol Psychiatry. 2024 Nov 15. doi: 10.1038/s41380-024-02831-y. Online ahead of print. Mol Psychiatry. 2024. PMID: 39543371
-
Embryonic Valproate Exposure Alters Mesencephalic Dopaminergic Neurons Distribution and Septal Dopaminergic Gene Expression in Domestic Chicks.Front Integr Neurosci. 2022 Mar 16;16:804881. doi: 10.3389/fnint.2022.804881. eCollection 2022. Front Integr Neurosci. 2022. PMID: 35369647 Free PMC article.
-
Head-to-head comparisons of carbon fiber microelectrode coatings for sensitive and selective neurotransmitter detection by voltammetry.Anal Chem. 2011 Sep 1;83(17):6658-66. doi: 10.1021/ac2011729. Epub 2011 Aug 3. Anal Chem. 2011. PMID: 21770471 Free PMC article.
-
Axonal and presynaptic FMRP: Localization, signal, and functional implications.Hear Res. 2023 Mar 15;430:108720. doi: 10.1016/j.heares.2023.108720. Epub 2023 Feb 11. Hear Res. 2023. PMID: 36809742 Free PMC article. Review.
-
RNA-binding proteins, neural development and the addictions.Genes Brain Behav. 2016 Jan;15(1):169-86. doi: 10.1111/gbb.12273. Genes Brain Behav. 2016. PMID: 26643147 Free PMC article. Review.
References
-
- Rogers S. J.; Wehner D. E.; Hagerman R. (2001) The behavioral phenotype in fragile X: symptoms of autism in very young children with fragile X syndrome, idiopathic autism, and other developmental disorders. J. Dev. Behav. Pediatr. 22, 409–417. - PubMed
-
- Feng Y.; Zhang F. P.; Lokey L. K.; Chastain J. L.; Lakkis L.; Eberhart D.; Warren S. T. (1995) Translational supprssion by trinucleotide repeat expansion at FMR1. Science 268, 731–734. - PubMed
-
- Oberle I.; Rousseau F.; Heitz D.; Kretz C.; Devys D.; Hanauer A.; Boue J.; Bertheas M. F.; Mandel J. L. (1991) Instability of a 550 base pair DNA segment and abnormal methylation in Fragile X Syndrome. Science 252, 1097–1102. - PubMed
-
- Cornish K.; Sudhalter V.; Turk J. (2004) Attention and language in fragile X. Ment. Retard. Dev. Disabil. Res. Rev. 10, 11–16. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

