Importance and regulation of the colonic mucus barrier in a mouse model of colitis
- PMID: 21109593
- PMCID: PMC3302190
- DOI: 10.1152/ajpgi.00422.2010
Importance and regulation of the colonic mucus barrier in a mouse model of colitis
Abstract
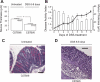
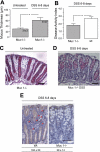
The colonic mucus layer serves as an important barrier and prevents colonic bacteria from invading the mucosa and cause inflammation. The regulation of colonic mucus secretion is poorly understood. The aim of this study was to investigate the role of the mucus barrier in induction of colitis. Furthermore, regulation of mucus secretion by luminal bacterial products was studied. The colon of anesthetized Muc2(-/-), Muc1(-/-), wild-type (wt), and germ-free mice was exteriorized, the mucosal surface was visualized, and mucus thickness was measured with micropipettes. Colitis was induced by DSS (dextran sodium sulfate, 3%, in drinking water), and disease activity index (DAI) was assessed daily. The colonic mucosa of germ-free and conventionally housed mice was exposed to the bacterial products LPS (lipopolysaccharide) and PGN (peptidoglycan). After DSS induction of colitis, the thickness of the firmly adherent mucus layer was significantly thinner after 5 days and onward, which paralleled the increment of DAI. Muc2(-/-) mice, which lacked firmly adherent mucus, were predisposed to colitis, whereas Muc1(-/-) mice were protected with significantly lower DAI by DSS compared with wt mice. The mucus barrier increased in Muc1(-/-) mice in response to DSS, whereas significantly fewer T cells were recruited to the inflamed colon. Mice housed under germ-free conditions had an extremely thin adherent colonic mucus layer, but when exposed to bacterial products (PGN or LPS) the thickness of the adherent mucus layer was quickly restored to levels observed in conventionally housed mice. This study demonstrates a correlation between decreasing mucus barrier and increasing clinical symptoms during onset of colitis. Mice lacking colonic mucus (Muc2(-/-)) were hypersensitive to DSS-induced colitis, whereas Muc1(-/-) were protected, probably through the ability to increase the mucus barrier but also by decreased T cell recruitment to the afflicted site. Furthermore, the ability of bacteria to regulate the thickness of the colonic mucus was demonstrated.
Figures


Similar articles
-
Loss of TMF/ARA160 protein renders colonic mucus refractory to bacterial colonization and diminishes intestinal susceptibility to acute colitis.J Biol Chem. 2012 Jul 20;287(30):25631-9. doi: 10.1074/jbc.M112.364786. Epub 2012 May 2. J Biol Chem. 2012. PMID: 22553199 Free PMC article.
-
Biochanin a ameliorates DSS-induced ulcerative colitis by improving colonic barrier function and protects against the development of spontaneous colitis in the Muc2 deficient mice.Chem Biol Interact. 2024 May 25;395:111014. doi: 10.1016/j.cbi.2024.111014. Epub 2024 Apr 20. Chem Biol Interact. 2024. PMID: 38648921
-
Compound sophorae decoction enhances intestinal barrier function of dextran sodium sulfate induced colitis via regulating notch signaling pathway in mice.Biomed Pharmacother. 2021 Jan;133:110937. doi: 10.1016/j.biopha.2020.110937. Epub 2020 Nov 17. Biomed Pharmacother. 2021. PMID: 33217689
-
MUC2 and related bacterial factors: Therapeutic targets for ulcerative colitis.EBioMedicine. 2021 Dec;74:103751. doi: 10.1016/j.ebiom.2021.103751. Epub 2021 Dec 10. EBioMedicine. 2021. PMID: 34902790 Free PMC article. Review.
-
Ant may well destroy a whole dam: glycans of colonic mucus barrier disintegrated by gut bacteria.Microbiol Res. 2024 Apr;281:127599. doi: 10.1016/j.micres.2023.127599. Epub 2024 Jan 4. Microbiol Res. 2024. PMID: 38219635 Review.
Cited by
-
Downregulation of hematopoietic MUC1 during experimental colitis increases tumor-promoting myeloid-derived suppressor cells.Clin Cancer Res. 2013 Sep 15;19(18):5039-52. doi: 10.1158/1078-0432.CCR-13-0278. Epub 2013 Jul 19. Clin Cancer Res. 2013. PMID: 23873692 Free PMC article.
-
Mucins in Intestinal Mucosal Defense and Inflammation: Learning From Clinical and Experimental Studies.Front Immunol. 2020 Sep 4;11:2054. doi: 10.3389/fimmu.2020.02054. eCollection 2020. Front Immunol. 2020. PMID: 33013869 Free PMC article. Review.
-
Intrarectal Capsazepine Administration Modulates Colonic Mucosal Health in Mice.Int J Mol Sci. 2022 Aug 24;23(17):9577. doi: 10.3390/ijms23179577. Int J Mol Sci. 2022. PMID: 36076974 Free PMC article.
-
Disruption of the gut microbiome as a risk factor for microbial infections.Curr Opin Microbiol. 2013 Apr;16(2):221-7. doi: 10.1016/j.mib.2013.03.009. Epub 2013 Apr 15. Curr Opin Microbiol. 2013. PMID: 23597788 Free PMC article. Review.
-
Integrity of the Intestinal Barrier: The Involvement of Epithelial Cells and Microbiota-A Mutual Relationship.Animals (Basel). 2022 Jan 8;12(2):145. doi: 10.3390/ani12020145. Animals (Basel). 2022. PMID: 35049768 Free PMC article. Review.
References
-
- Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol 10: 131–144, 2010 - PubMed
-
- Ahn DH, Crawley SC, Hokari R, Kato S, Yang SC, Li JD, Kim YS. TNF-alpha activates MUC2 transcription via NF-kappaB but inhibits via JNK activation. Cell Physiol Biochem 15: 29–40, 2005 - PubMed
-
- Atuma C, Strugala V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am J Physiol Gastrointest Liver Physiol 280: G922–G929, 2001 - PubMed
-
- Backert S, Selbach M. Role of type IV secretion in Helicobacter pylori pathogenesis. Cell Microbiol 10: 1573–1581, 2008 - PubMed
-
- Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science 307: 1915–1920, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

