Nonconventional CD8+ T cell responses to Listeria infection in mice lacking MHC class Ia and H2-M3
- PMID: 21098224
- PMCID: PMC3068915
- DOI: 10.4049/jimmunol.1002639
Nonconventional CD8+ T cell responses to Listeria infection in mice lacking MHC class Ia and H2-M3
Abstract
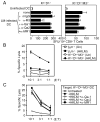
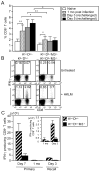
CD8(+) T cells restricted to MHC class Ib molecules other than H2-M3 have been shown to recognize bacterial Ags. However, the contribution of these T cells to immune responses against bacterial infection is not well defined. To investigate the immune potential of MHC class Ib-restricted CD8(+) T cells, we have generated mice that lack both MHC class Ia and H2-M3 molecules (K(b-/-)D (b-/-)M3(-/-)). The CD8(+) T cells present in K(b-/-)D (b-/-)M3(-/-) mice display an activated surface phenotype and are able to secrete IFN-γ rapidly upon anti-CD3 and anti-CD28 stimulation. Although the CD8(+) T cell population is reduced in K(b-/-)D (b-/-)M3(-/-) mice compared with that in K(b-/-)D (b-/-) mice, this population retains the capacity to expand significantly in response to primary infection with the bacteria Listeria monocytogenes. However, K(b-/-)D (b-/-)M3(-/-) CD8(+) T cells do not expand upon secondary infection, similar to what has been observed for H2-M3-restricted T cells. CD8(+) T cells isolated from Listeria-infected K(b-/-)D (b-/-)M3(-/-) mice exhibit cytotoxicity and secrete proinflammatory cytokines in response to Listeria-infected APCs. These T cells are protective against primary Listeria infection, as Listeria-infected K(b-/-)D (b-/-)M3(-/-) mice exhibit reduced bacterial burden compared with that of infected β(2)-microglobulin-deficient mice that lack MHC class Ib-restricted CD8(+) T cells altogether. In addition, adoptive transfer of Listeria-experienced K(b-/-)D (b-/-)M3(-/-) splenocytes protects recipient mice against subsequent Listeria infection in a CD8(+) T cell-dependent manner. These data demonstrate that other MHC class Ib-restricted CD8(+) T cells, in addition to H2-M3-restricted T cells, contribute to antilisterial immunity and may contribute to immune responses against other intracellular bacteria.
Figures
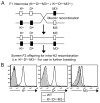
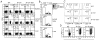
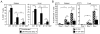
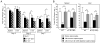
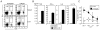
Similar articles
-
Class Ia MHC-deficient BALB/c mice generate CD8+ T cell-mediated protective immunity against Listeria monocytogenes infection.J Immunol. 2003 Jul 1;171(1):291-8. doi: 10.4049/jimmunol.171.1.291. J Immunol. 2003. PMID: 12817010
-
MHC class Ib-restricted CTL provide protection against primary and secondary Listeria monocytogenes infection.J Immunol. 2000 Nov 1;165(9):5192-201. doi: 10.4049/jimmunol.165.9.5192. J Immunol. 2000. PMID: 11046052
-
H2-M3-restricted CD8+ T cells are not required for MHC class Ib-restricted immunity against Listeria monocytogenes.J Exp Med. 2006 Feb 20;203(2):383-91. doi: 10.1084/jem.20052256. Epub 2006 Feb 6. J Exp Med. 2006. PMID: 16461341 Free PMC article.
-
Role of MHC class Ib molecule, H2-M3 in host immunity against tuberculosis.Vaccine. 2013 Aug 20;31(37):3818-25. doi: 10.1016/j.vaccine.2013.04.005. Epub 2013 Apr 28. Vaccine. 2013. PMID: 23628242 Review.
-
CTL responses to H2-M3-restricted Listeria epitopes.Immunol Rev. 1997 Aug;158:115-21. doi: 10.1111/j.1600-065x.1997.tb00997.x. Immunol Rev. 1997. PMID: 9314079 Review.
Cited by
-
Nonclassical MHC Ib-restricted CD8+ T Cells Recognize Mycobacterium tuberculosis-Derived Protein Antigens and Contribute to Protection Against Infection.PLoS Pathog. 2016 Jun 7;12(6):e1005688. doi: 10.1371/journal.ppat.1005688. eCollection 2016 Jun. PLoS Pathog. 2016. PMID: 27272249 Free PMC article.
-
Expression of the mouse MHC class Ib H2-T11 gene product, a paralog of H2-T23 (Qa-1) with shared peptide-binding specificity.J Immunol. 2014 Aug 1;193(3):1427-39. doi: 10.4049/jimmunol.1302048. Epub 2014 Jun 23. J Immunol. 2014. PMID: 24958902 Free PMC article.
-
An MHC class Ib-restricted CD8+ T cell response to lymphocytic choriomeningitis virus.J Immunol. 2011 Dec 15;187(12):6463-72. doi: 10.4049/jimmunol.1101171. Epub 2011 Nov 14. J Immunol. 2011. PMID: 22084437 Free PMC article.
-
Early events regulating immunity and pathogenesis during Listeria monocytogenes infection.Trends Immunol. 2012 Oct;33(10):488-95. doi: 10.1016/j.it.2012.04.007. Epub 2012 Jun 5. Trends Immunol. 2012. PMID: 22677184 Free PMC article. Review.
-
Positive selecting cell type determines the phenotype of MHC class Ib-restricted CD8+ T cells.Proc Natl Acad Sci U S A. 2011 Aug 9;108(32):13241-6. doi: 10.1073/pnas.1105118108. Epub 2011 Jul 25. Proc Natl Acad Sci U S A. 2011. PMID: 21788511 Free PMC article.
References
-
- Rodgers JR, Cook RG. MHC class Ib molecules bridge innate and acquired immunity. Nat Rev Immunol. 2005;5:459–471. - PubMed
-
- Jensen PE, Sullivan BA, Reed-Loisel LM, Weber DA. Qa-1, a nonclassical class I histocompatibility molecule with roles in innate and adaptive immunity. Immunol Res. 2004;29:81–92. - PubMed
-
- Dascher CC. Evolutionary biology of CD1. Curr Top Microbiol Immunol. 2007;314:3–26. - PubMed
-
- Howcroft TK, Singer DS. Expression of nonclassical MHC class Ib genes: comparison of regulatory elements. Immunol Res. 2003;27:1–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

