Development and characterization of a highly specific and sensitive SYBR green reverse transcriptase PCR assay for detection of the 2009 pandemic H1N1 influenza virus on the basis of sequence signatures
- PMID: 21084522
- PMCID: PMC3020443
- DOI: 10.1128/JCM.01142-10
Development and characterization of a highly specific and sensitive SYBR green reverse transcriptase PCR assay for detection of the 2009 pandemic H1N1 influenza virus on the basis of sequence signatures
Abstract
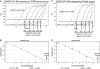
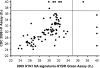
The emergence and rapid spread of the 2009 H1N1 pandemic influenza virus showed that many diagnostic tests were unsuitable for detecting the novel virus isolates. In most countries the probe-based TaqMan assay developed by the U.S. Centers for Disease Control and Prevention was used for diagnostic purposes. The substantial sequence data that became available during the course of the pandemic created the opportunity to utilize bioinformatics tools to evaluate the unique sequence properties of this virus for the development of diagnostic tests. We used a comprehensive computational approach to examine conserved 2009 H1N1 sequence signatures that are at least 20 nucleotides long and contain at least two mismatches compared to any other known H1N1 genome. We found that the hemagglutinin (HA) and neuraminidase (NA) genes contained sequence signatures that are highly conserved among 2009 H1N1 isolates. Based on the NA gene signatures, we used Visual-OMP to design primers with optimal hybridization affinity and we used ThermoBLAST to minimize amplification artifacts. This procedure resulted in a highly sensitive and discriminatory 2009 H1N1 detection assay. Importantly, we found that the primer set can be used reliably in both a conventional TaqMan and a SYBR green reverse transcriptase (RT)-PCR assay with no loss of specificity or sensitivity. We validated the diagnostic accuracy of the NA SYBR green assay with 125 clinical specimens obtained between May and August 2009 in Chile, and we showed diagnostic efficacy comparable to the CDC assay. Our approach highlights the use of systematic computational approaches to develop robust diagnostic tests during a viral pandemic.
Figures

Similar articles
-
Evaluation of twelve real-time reverse transcriptase PCR primer-probe sets for detection of pandemic influenza A/H1N1 2009 virus.J Clin Microbiol. 2011 Apr;49(4):1434-40. doi: 10.1128/JCM.01914-10. Epub 2011 Feb 2. J Clin Microbiol. 2011. PMID: 21289144 Free PMC article.
-
Library of prefabricated locked nucleic acid hydrolysis probes facilitates rapid development of reverse-transcription quantitative real-time PCR assays for detection of novel influenza A/H1N1/09 virus.Clin Chem. 2009 Dec;55(12):2218-22. doi: 10.1373/clinchem.2009.136192. Epub 2009 Oct 1. Clin Chem. 2009. PMID: 19797710
-
Evaluation of a rapid molecular algorithm for detection of pandemic influenza A (H1N1) 2009 virus and screening for a key oseltamivir resistance (H275Y) substitution in neuraminidase.J Clin Virol. 2010 Jan;47(1):34-7. doi: 10.1016/j.jcv.2009.09.030. Epub 2009 Oct 25. J Clin Virol. 2010. PMID: 19857993 Free PMC article.
-
Tools to detect influenza virus.Yonsei Med J. 2013 May 1;54(3):560-6. doi: 10.3349/ymj.2013.54.3.560. Yonsei Med J. 2013. PMID: 23549796 Free PMC article. Review.
-
Update on influenza diagnostics: lessons from the novel H1N1 influenza A pandemic.Clin Microbiol Rev. 2012 Apr;25(2):344-61. doi: 10.1128/CMR.05016-11. Clin Microbiol Rev. 2012. PMID: 22491775 Free PMC article. Review.
Cited by
-
T-cell-mediated cross-strain protective immunity elicited by prime-boost vaccination with a live attenuated influenza vaccine.Int J Infect Dis. 2014 Oct;27:37-43. doi: 10.1016/j.ijid.2014.05.016. Epub 2014 Aug 27. Int J Infect Dis. 2014. PMID: 25172265 Free PMC article.
-
Rapid detection of the H275Y oseltamivir resistance mutation in influenza A/H1N1 2009 by single base pair RT-PCR and high-resolution melting.PLoS One. 2011;6(6):e21446. doi: 10.1371/journal.pone.0021446. Epub 2011 Jun 24. PLoS One. 2011. PMID: 21731753 Free PMC article.
-
Fast Evaluation of Viral Emerging Risks (FEVER): A computational tool for biosurveillance, diagnostics, and mutation typing of emerging viral pathogens.PLOS Glob Public Health. 2022 Feb 24;2(2):e0000207. doi: 10.1371/journal.pgph.0000207. eCollection 2022. PLOS Glob Public Health. 2022. PMID: 36962401 Free PMC article.
-
Assays for monitoring susceptibility of influenza viruses to neuraminidase inhibitors.Influenza Other Respir Viruses. 2013 Jan;7 Suppl 1(Suppl 1):44-9. doi: 10.1111/irv.12051. Influenza Other Respir Viruses. 2013. PMID: 23279896 Free PMC article. Review.
-
A quantitative fluorescence-based steady-state assay of DNA polymerase.FEBS J. 2014 Apr;281(8):2042-50. doi: 10.1111/febs.12760. FEBS J. 2014. PMID: 24860875 Free PMC article.
References
-
- Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. - PubMed
-
- Centers for Disease Control and Prevention. 2009. Evaluation of rapid influenza diagnostic tests for detection of novel influenza A (H1N1) virus—United States, 2009. MMWR Morb. Mortal. Wkly. Rep. 58:826-829. - PubMed
-
- Centers for Disease Control and Prevention. 2009. Human infection with new influenza A (H1N1) virus: clinical observations from Mexico and other affected countries, May 2009. Wkly. Epidemiol. Rec. 84:185-189. - PubMed
-
- Centers for Disease Control and Prevention. 2009. Outbreak of swine-origin influenza A (H1N1) virus infection—Mexico, March-April 2009. MMWR Morb. Mortal. Wkly. Rep. 58:467-470. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

