Cloning of the Epstein-Barr virus-related rhesus lymphocryptovirus as a bacterial artificial chromosome: a loss-of-function mutation of the rhBARF1 immune evasion gene
- PMID: 21084476
- PMCID: PMC3020515
- DOI: 10.1128/JVI.01411-10
Cloning of the Epstein-Barr virus-related rhesus lymphocryptovirus as a bacterial artificial chromosome: a loss-of-function mutation of the rhBARF1 immune evasion gene
Abstract
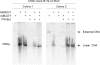
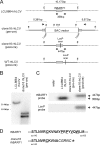
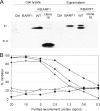
Rhesus macaques are naturally infected with a gammaherpesvirus which is in the same lymphocryptovirus (LCV) genus as and closely related to Epstein-Barr virus (EBV). The rhesus macaque LCV (rhLCV) contains a repertoire of genes identical to that of EBV, and experimental rhLCV infection of naive rhesus macaques accurately models acute and persistent EBV infection of humans. We cloned the LCL8664 rhLCV strain as a bacterial artificial chromosome to create recombinant rhLCV for investigation in this animal model system. A recombinant rhLCV (clone 16 rhLCV) carrying a mutation in the putative immune evasion gene rhBARF1 was created along with a rescued wild-type (rWT) rhLCV in which the rhBARF1 open reading frame (ORF) was repaired. The rWT rhLCV molecular clone demonstrated viral replication and B-cell immortalization properties comparable to those of the naturally derived LCL8664 rhLCV. Qualitatively, clone 16 rhLCV carrying a mutated rhBARF1 was competent for viral replication and B-cell immortalization, but quantitative assays showed that clone 16 rhLCV immortalized B cells less efficiently than LCL8664 and rWT rhLCV. Functional studies showed that rhBARF1 could block CSF-1 cytokine signaling as well as EBV BARF1, whereas the truncated rhBARF1 from clone 16 rhLCV was a loss-of-function mutant. These recombinant rhLCV can be used in the rhesus macaque animal model system to better understand how a putative viral immune evasion gene contributes to the pathogenesis of acute and persistent EBV infection. The development of a genetic system for making recombinant rhLCV constitutes a major advance in the study of EBV pathogenesis in the rhesus macaque animal model.
Figures


Similar articles
-
An Epstein-Barr virus encoded inhibitor of Colony Stimulating Factor-1 signaling is an important determinant for acute and persistent EBV infection.PLoS Pathog. 2012 Dec;8(12):e1003095. doi: 10.1371/journal.ppat.1003095. Epub 2012 Dec 27. PLoS Pathog. 2012. PMID: 23300447 Free PMC article.
-
Epstein-Barr Virus gp350 Can Functionally Replace the Rhesus Lymphocryptovirus Major Membrane Glycoprotein and Does Not Restrict Infection of Rhesus Macaques.J Virol. 2015 Nov 11;90(3):1222-30. doi: 10.1128/JVI.02531-15. Print 2016 Feb 1. J Virol. 2015. PMID: 26559839 Free PMC article.
-
Antibodies to lytic infection proteins in lymphocryptovirus-infected rhesus macaques: a model for humoral immune responses to epstein-barr virus infection.Clin Vaccine Immunol. 2011 Sep;18(9):1427-34. doi: 10.1128/CVI.05126-11. Epub 2011 Jul 6. Clin Vaccine Immunol. 2011. PMID: 21734064 Free PMC article.
-
Non-human Primate Lymphocryptoviruses: Past, Present, and Future.Curr Top Microbiol Immunol. 2015;391:385-405. doi: 10.1007/978-3-319-22834-1_13. Curr Top Microbiol Immunol. 2015. PMID: 26428382 Review.
-
Nonhuman primate models for Epstein-Barr virus infection.Curr Opin Virol. 2013 Jun;3(3):233-7. doi: 10.1016/j.coviro.2013.03.003. Epub 2013 Apr 3. Curr Opin Virol. 2013. PMID: 23562212 Free PMC article. Review.
Cited by
-
Animal models of tumorigenic herpesviruses--an update.Curr Opin Virol. 2015 Oct;14:145-50. doi: 10.1016/j.coviro.2015.09.006. Curr Opin Virol. 2015. PMID: 26476352 Free PMC article. Review.
-
Multipronged attenuation of macrophage-colony stimulating factor signaling by Epstein-Barr virus BARF1.Proc Natl Acad Sci U S A. 2012 Aug 7;109(32):12962-7. doi: 10.1073/pnas.1205309109. Epub 2012 Jul 23. Proc Natl Acad Sci U S A. 2012. PMID: 22826234 Free PMC article.
-
Allosteric competitive inactivation of hematopoietic CSF-1 signaling by the viral decoy receptor BARF1.Nat Struct Mol Biol. 2012 Sep;19(9):938-47. doi: 10.1038/nsmb.2367. Epub 2012 Aug 19. Nat Struct Mol Biol. 2012. PMID: 22902366
-
BamHI-A rightward frame 1, an Epstein-Barr virus-encoded oncogene and immune modulator.Rev Med Virol. 2013 Nov;23(6):367-83. doi: 10.1002/rmv.1758. Epub 2013 Aug 31. Rev Med Virol. 2013. PMID: 23996634 Free PMC article. Review.
-
Nonhuman primate models of human viral infections.Nat Rev Immunol. 2018 Jun;18(6):390-404. doi: 10.1038/s41577-018-0005-7. Nat Rev Immunol. 2018. PMID: 29556017 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

