The N-end rule pathway is mediated by a complex of the RING-type Ubr1 and HECT-type Ufd4 ubiquitin ligases
- PMID: 21076411
- PMCID: PMC3003441
- DOI: 10.1038/ncb2121
The N-end rule pathway is mediated by a complex of the RING-type Ubr1 and HECT-type Ufd4 ubiquitin ligases
Abstract
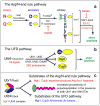
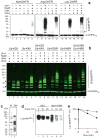
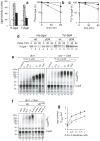
Substrates of the N-end rule pathway are recognized by the Ubr1 E3 ubiquitin ligase through their destabilizing amino-terminal residues. Our previous work showed that the Ubr1 E3 and the Ufd4 E3 together target an internal degradation signal (degron) of the Mgt1 DNA repair protein. Ufd4 is an E3 enzyme of the ubiquitin-fusion degradation (UFD) pathway that recognizes an N-terminal ubiquitin moiety. Here we show that the RING-type Ubr1 E3 and the HECT-type Ufd4 E3 interact, both physically and functionally. Although Ubr1 can recognize and polyubiquitylate an N-end rule substrate in the absence of Ufd4, the Ubr1-Ufd4 complex is more processive in that it produces a longer substrate-linked polyubiquitin chain. Conversely, Ubr1 can function as a polyubiquitylation-enhancing component of the Ubr1-Ufd4 complex in its targeting of UFD substrates. We also found that Ubr1 can recognize the N-terminal ubiquitin moiety. These and related advances unify two proteolytic systems that have been studied separately for two decades.
Conflict of interest statement
The authors declare that they have no competing financial interest.
Figures



Comment in
-
Working on a chain: E3s ganging up for ubiquitylation.Nat Cell Biol. 2010 Dec;12(12):1124-6. doi: 10.1038/ncb1210-1124. Nat Cell Biol. 2010. PMID: 21124306
-
Ubiquitylation: E3 ligases team up.Nat Rev Mol Cell Biol. 2011 Jan;12(1):6. doi: 10.1038/nrm3033. Epub 2010 Dec 8. Nat Rev Mol Cell Biol. 2011. PMID: 21139637 No abstract available.
Similar articles
-
Five enzymes of the Arg/N-degron pathway form a targeting complex: The concept of superchanneling.Proc Natl Acad Sci U S A. 2020 May 19;117(20):10778-10788. doi: 10.1073/pnas.2003043117. Epub 2020 May 4. Proc Natl Acad Sci U S A. 2020. PMID: 32366662 Free PMC article.
-
Substrate-binding sites of UBR1, the ubiquitin ligase of the N-end rule pathway.J Biol Chem. 2008 Aug 29;283(35):24011-28. doi: 10.1074/jbc.M802583200. Epub 2008 Jun 19. J Biol Chem. 2008. PMID: 18566452 Free PMC article.
-
Pairs of dipeptides synergistically activate the binding of substrate by ubiquitin ligase through dissociation of its autoinhibitory domain.Proc Natl Acad Sci U S A. 2002 Oct 29;99(22):14110-5. doi: 10.1073/pnas.172527399. Epub 2002 Oct 21. Proc Natl Acad Sci U S A. 2002. PMID: 12391316 Free PMC article.
-
Proteolytic regulation of metabolic enzymes by E3 ubiquitin ligase complexes: lessons from yeast.Crit Rev Biochem Mol Biol. 2015;50(6):489-502. doi: 10.3109/10409238.2015.1081869. Epub 2015 Sep 11. Crit Rev Biochem Mol Biol. 2015. PMID: 26362128 Review.
-
Orchestra for assembly and fate of polyubiquitin chains.Essays Biochem. 2005;41:1-14. doi: 10.1042/EB0410001. Essays Biochem. 2005. PMID: 16250894 Review.
Cited by
-
hecd-1 modulates notch activity in Caenorhabditis elegans.G3 (Bethesda). 2014 Dec 31;5(3):353-9. doi: 10.1534/g3.114.015321. G3 (Bethesda). 2014. PMID: 25552605 Free PMC article.
-
The N-end rule pathway counteracts cell death by destroying proapoptotic protein fragments.Proc Natl Acad Sci U S A. 2012 Jul 3;109(27):E1839-47. doi: 10.1073/pnas.1207786109. Epub 2012 Jun 5. Proc Natl Acad Sci U S A. 2012. PMID: 22670058 Free PMC article.
-
Crystal structure of the Ate1 arginyl-tRNA-protein transferase and arginylation of N-degron substrates.Proc Natl Acad Sci U S A. 2022 Aug 2;119(31):e2209597119. doi: 10.1073/pnas.2209597119. Epub 2022 Jul 25. Proc Natl Acad Sci U S A. 2022. PMID: 35878037 Free PMC article.
-
Ubiquitin Ligase Redundancy and Nuclear-Cytoplasmic Localization in Yeast Protein Quality Control.Biomolecules. 2021 Dec 3;11(12):1821. doi: 10.3390/biom11121821. Biomolecules. 2021. PMID: 34944465 Free PMC article. Review.
-
Regulating the human HECT E3 ligases.Cell Mol Life Sci. 2018 Sep;75(17):3121-3141. doi: 10.1007/s00018-018-2848-2. Epub 2018 Jun 1. Cell Mol Life Sci. 2018. PMID: 29858610 Free PMC article. Review.
References
-
- Bachmair A, Finley D, Varshavsky A. In vivo half-life of a protein is a function of its amino-terminal residue. Science. 1986;234:179–186. - PubMed
-
- Turner GC, Du F, Varshavsky A. Peptides accelerate their uptake by activating a ubiquitin-dependent proteolytic pathway. Nature. 2000;405:579–583. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK039520-22A1/DK/NIDDK NIH HHS/United States
- R01 DK039520-24/DK/NIDDK NIH HHS/United States
- GM085371/GM/NIGMS NIH HHS/United States
- R01 DK039520-23/DK/NIDDK NIH HHS/United States
- R01 GM031530/GM/NIGMS NIH HHS/United States
- R01 DK039520/DK/NIDDK NIH HHS/United States
- R01 DK039520-22A1S1/DK/NIDDK NIH HHS/United States
- DK039520/DK/NIDDK NIH HHS/United States
- R37 DK039520/DK/NIDDK NIH HHS/United States
- R01 GM031530-29/GM/NIGMS NIH HHS/United States
- GM031530/GM/NIGMS NIH HHS/United States
- R01 GM085371/GM/NIGMS NIH HHS/United States
- R01 GM085371-03/GM/NIGMS NIH HHS/United States
- R56 DK039520/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

