Inorganic nanoparticle-based contrast agents for molecular imaging
- PMID: 21074494
- PMCID: PMC3052982
- DOI: 10.1016/j.molmed.2010.09.004
Inorganic nanoparticle-based contrast agents for molecular imaging
Abstract
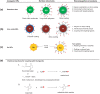
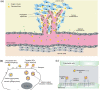
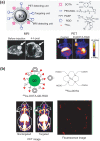
Inorganic nanoparticles (NPs) including semiconductor quantum dots (QDs), iron oxide NPs and gold NPs have been developed as contrast agents for diagnostics by molecular imaging. Compared with traditional contrast agents, NPs offer several advantages: their optical and magnetic properties can be tailored by engineering the composition, structure, size and shape; their surfaces can be modified with ligands to target specific biomarkers of disease; the contrast enhancement provided can be equivalent to millions of molecular counterparts; and they can be integrated with a combination of different functions for multimodal imaging. Here, we review recent advances in the development of contrast agents based on inorganic NPs for molecular imaging, and also touch on contrast enhancement, surface modification, tissue targeting, clearance and toxicity. As research efforts intensify, contrast agents based on inorganic NPs that are highly sensitive, target-specific and safe to use are expected to enter clinical applications in the near future.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Inorganic Nanoparticles for Cancer Therapy: A Transition from Lab to Clinic.Curr Med Chem. 2018;25(34):4269-4303. doi: 10.2174/0929867325666171229141156. Curr Med Chem. 2018. PMID: 29284391 Review.
-
Functionalization of inorganic nanoparticles for bioimaging applications.Acc Chem Res. 2011 Oct 18;44(10):925-35. doi: 10.1021/ar2000327. Epub 2011 Jun 7. Acc Chem Res. 2011. PMID: 21648430 Review.
-
Nanoparticulate Contrast Agents for Multimodality Molecular Imaging.J Biomed Nanotechnol. 2016 Aug;12(8):1553-84. doi: 10.1166/jbn.2016.2258. J Biomed Nanotechnol. 2016. PMID: 29341579 Review.
-
Surface engineering of inorganic nanoparticles for imaging and therapy.Adv Drug Deliv Rev. 2013 May;65(5):622-48. doi: 10.1016/j.addr.2012.08.015. Epub 2012 Sep 6. Adv Drug Deliv Rev. 2013. PMID: 22975010 Review.
-
Nanoparticles in practice for molecular-imaging applications: An overview.Acta Biomater. 2016 Sep 1;41:1-16. doi: 10.1016/j.actbio.2016.06.003. Epub 2016 Jun 2. Acta Biomater. 2016. PMID: 27265153 Review.
Cited by
-
Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy.Theranostics. 2012;2(1):3-44. doi: 10.7150/thno.3463. Epub 2012 Jan 1. Theranostics. 2012. PMID: 22272217 Free PMC article.
-
Computed tomography imaging of macrophage phagocytic activity in abdominal aortic aneurysm.Theranostics. 2021 Apr 3;11(12):5876-5888. doi: 10.7150/thno.55106. eCollection 2021. Theranostics. 2021. PMID: 33897887 Free PMC article.
-
Nonviral Delivery Systems of mRNA Vaccines for Cancer Gene Therapy.Pharmaceutics. 2022 Feb 25;14(3):512. doi: 10.3390/pharmaceutics14030512. Pharmaceutics. 2022. PMID: 35335891 Free PMC article. Review.
-
Exogenous Contrast Agents in Photoacoustic Imaging: An In Vivo Review for Tumor Imaging.Nanomaterials (Basel). 2022 Jan 25;12(3):393. doi: 10.3390/nano12030393. Nanomaterials (Basel). 2022. PMID: 35159738 Free PMC article. Review.
-
High-throughput quantitation of inorganic nanoparticle biodistribution at the single-cell level using mass cytometry.Nat Commun. 2017 Jan 17;8:14069. doi: 10.1038/ncomms14069. Nat Commun. 2017. PMID: 28094297 Free PMC article.
References
-
- Weissleder R, Mahmood U. Molecular imaging. Radiology. 2001;219:316–333. - PubMed
-
- Achilefu S. Introduction to concept and strategies for molecular imaging. Chem. Rev. 2010;110:2575–2578. - PubMed
-
- Licha K. Contrast agents for optical imaging. Topics Curr. Chem. 2002;222:1–29.
-
- Gao X, et al. In vivo molecular and cellular imaging with quantum dots. Curr. Opin. Biotechnol. 2005;16:63–72. - PubMed
-
- Erdi YM. The use of PET for radiotherapy. Curr. Medical Imaging Rev. 2007;3:3–16.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

