Structural studies of the tandem Tudor domains of fragile X mental retardation related proteins FXR1 and FXR2
- PMID: 21072162
- PMCID: PMC2970552
- DOI: 10.1371/journal.pone.0013559
Structural studies of the tandem Tudor domains of fragile X mental retardation related proteins FXR1 and FXR2
Abstract
Background: Expansion of the CGG trinucleotide repeat in the 5'-untranslated region of the FMR1, fragile X mental retardation 1, gene results in suppression of protein expression for this gene and is the underlying cause of Fragile X syndrome. In unaffected individuals, the FMRP protein, together with two additional paralogues (Fragile X Mental Retardation Syndrome-related Protein 1 and 2), associates with mRNA to form a ribonucleoprotein complex in the nucleus that is transported to dendrites and spines of neuronal cells. It is thought that the fragile X family of proteins contributes to the regulation of protein synthesis at sites where mRNAs are locally translated in response to stimuli.
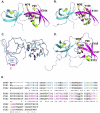
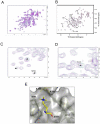
Methodology/principal findings: Here, we report the X-ray crystal structures of the non-canonical nuclear localization signals of the FXR1 and FXR2 autosomal paralogues of FMRP, which were determined at 2.50 and 1.92 Å, respectively. The nuclear localization signals of the FXR1 and FXR2 comprise tandem Tudor domain architectures, closely resembling that of UHRF1, which is proposed to bind methylated histone H3K9.
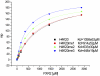
Conclusions: The FMRP, FXR1 and FXR2 proteins comprise a small family of highly conserved proteins that appear to be important in translational regulation, particularly in neuronal cells. The crystal structures of the N-terminal tandem Tudor domains of FXR1 and FXR2 revealed a conserved architecture with that of FMRP. Biochemical analysis of the tandem Tudor domains reveals their ability to preferentially recognize trimethylated peptides in a sequence-specific manner.
Enhanced version: This article can also be viewed as an enhanced version in which the text of the article is integrated with interactive 3D representations and animated transitions. Please note that a web plugin is required to access this enhanced functionality. Instructions for the installation and use of the web plugin are available in Text S1.
Conflict of interest statement
Figures

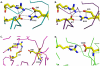
Similar articles
-
Structural and histone binding ability characterizations of human PWWP domains.PLoS One. 2011;6(6):e18919. doi: 10.1371/journal.pone.0018919. Epub 2011 Jun 20. PLoS One. 2011. PMID: 21720545 Free PMC article.
-
Tdrd3 is a novel stress granule-associated protein interacting with the Fragile-X syndrome protein FMRP.Hum Mol Genet. 2008 Oct 15;17(20):3236-46. doi: 10.1093/hmg/ddn219. Epub 2008 Jul 28. Hum Mol Genet. 2008. PMID: 18664458
-
The fragile X mental retardation syndrome protein interacts with novel homologs FXR1 and FXR2.EMBO J. 1995 Nov 1;14(21):5358-66. doi: 10.1002/j.1460-2075.1995.tb00220.x. EMBO J. 1995. PMID: 7489725 Free PMC article.
-
Fragile X-related protein family: a double-edged sword in neurodevelopmental disorders and cancer.Crit Rev Biochem Mol Biol. 2020 Oct;55(5):409-424. doi: 10.1080/10409238.2020.1810621. Epub 2020 Sep 2. Crit Rev Biochem Mol Biol. 2020. PMID: 32878499 Free PMC article. Review.
-
The Fragile X mental retardation protein.Brain Res Bull. 2001 Oct-Nov 1;56(3-4):375-82. doi: 10.1016/s0361-9230(01)00647-5. Brain Res Bull. 2001. PMID: 11719275 Review.
Cited by
-
Fragile X mental retardation protein: A paradigm for translational control by RNA-binding proteins.Biochimie. 2015 Jul;114:147-54. doi: 10.1016/j.biochi.2015.02.005. Epub 2015 Feb 17. Biochimie. 2015. PMID: 25701550 Free PMC article. Review.
-
Fmr1 KO and fenobam treatment differentially impact distinct synapse populations of mouse neocortex.Neuron. 2014 Dec 17;84(6):1273-86. doi: 10.1016/j.neuron.2014.11.016. Neuron. 2014. PMID: 25521380 Free PMC article.
-
PHF20 is an effector protein of p53 double lysine methylation that stabilizes and activates p53.Nat Struct Mol Biol. 2012 Sep;19(9):916-24. doi: 10.1038/nsmb.2353. Epub 2012 Aug 5. Nat Struct Mol Biol. 2012. PMID: 22864287 Free PMC article.
-
Drosophila melanogaster as a Model to Study the Multiple Phenotypes, Related to Genome Stability of the Fragile-X Syndrome.Front Genet. 2019 Feb 13;10:10. doi: 10.3389/fgene.2019.00010. eCollection 2019. Front Genet. 2019. PMID: 30815010 Free PMC article. Review.
-
SAFlex: A structural alphabet extension to integrate protein structural flexibility and missing data information.PLoS One. 2018 Jul 5;13(7):e0198854. doi: 10.1371/journal.pone.0198854. eCollection 2018. PLoS One. 2018. PMID: 29975698 Free PMC article.
References
-
- Turner G, Webb T, Wake S, Robinson H. Prevalence of fragile X syndrome. Am J Med Genet. 1996;64:196–197. - PubMed
-
- Warren ST, Nelson DL. Advances in molecular analysis of fragile X syndrome. JAMA. 1994;271:536–542. - PubMed
-
- Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. - PubMed
-
- Kenneson A, Zhang F, Hagedorn CH, Warren ST. Reduced FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. Hum Mol Genet. 2001;10:1449–1454. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

