Regulation of synaptic connectivity by glia
- PMID: 21068831
- PMCID: PMC4431554
- DOI: 10.1038/nature09612
Regulation of synaptic connectivity by glia
Abstract
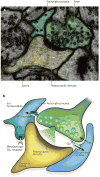
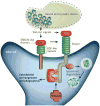
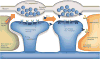
The human brain contains more than 100 trillion (10(14)) synaptic connections, which form all of its neural circuits. Neuroscientists have long been interested in how this complex synaptic web is weaved during development and remodelled during learning and disease. Recent studies have uncovered that glial cells are important regulators of synaptic connectivity. These cells are far more active than was previously thought and are powerful controllers of synapse formation, function, plasticity and elimination, both in health and disease. Understanding how signalling between glia and neurons regulates synaptic development will offer new insight into how the nervous system works and provide new targets for the treatment of neurological diseases.
Figures

Similar articles
-
Glial cells in synaptic plasticity.J Physiol Paris. 2006 Mar-May;99(2-3):75-83. doi: 10.1016/j.jphysparis.2005.12.002. Epub 2006 Jan 30. J Physiol Paris. 2006. PMID: 16446078 Review.
-
Roles of glial cells in synapse development.Cell Mol Life Sci. 2009 Jul;66(13):2037-47. doi: 10.1007/s00018-009-0005-7. Epub 2009 Mar 24. Cell Mol Life Sci. 2009. PMID: 19308323 Free PMC article. Review.
-
Signaling between glia and neurons: focus on synaptic plasticity.Curr Opin Neurobiol. 2005 Oct;15(5):542-8. doi: 10.1016/j.conb.2005.08.006. Curr Opin Neurobiol. 2005. PMID: 16144764 Review.
-
Role of neuron-glia interactions in developmental synapse elimination.Brain Res Bull. 2017 Mar;129:74-81. doi: 10.1016/j.brainresbull.2016.08.017. Epub 2016 Sep 4. Brain Res Bull. 2017. PMID: 27601093 Review.
-
Glia as architects of central nervous system formation and function.Science. 2018 Oct 12;362(6411):181-185. doi: 10.1126/science.aat0473. Science. 2018. PMID: 30309945 Free PMC article. Review.
Cited by
-
Primary cilia signaling in astrocytes mediates development and regional-specific functional specification.Nat Neurosci. 2024 Sep;27(9):1708-1720. doi: 10.1038/s41593-024-01726-z. Epub 2024 Aug 5. Nat Neurosci. 2024. PMID: 39103557
-
In vivo metabolic labeling of sialoglycans in the mouse brain by using a liposome-assisted bioorthogonal reporter strategy.Proc Natl Acad Sci U S A. 2016 May 10;113(19):5173-8. doi: 10.1073/pnas.1516524113. Epub 2016 Apr 28. Proc Natl Acad Sci U S A. 2016. PMID: 27125855 Free PMC article.
-
Suppression of a MEF2-KLF6 survival pathway by PKA signaling promotes apoptosis in embryonic hippocampal neurons.J Neurosci. 2012 Feb 22;32(8):2790-803. doi: 10.1523/JNEUROSCI.3609-11.2012. J Neurosci. 2012. PMID: 22357862 Free PMC article.
-
Voluntary Exercise Induces Astrocytic Structural Plasticity in the Globus Pallidus.Front Cell Neurosci. 2016 Jun 21;10:165. doi: 10.3389/fncel.2016.00165. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27445692 Free PMC article.
-
13C NMR metabolomic evaluation of immediate and delayed mild hypothermia in cerebrocortical slices after oxygen-glucose deprivation.Anesthesiology. 2013 Nov;119(5):1120-36. doi: 10.1097/ALN.0b013e31829c2d90. Anesthesiology. 2013. PMID: 23748856 Free PMC article.
References
-
- Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60:430–440. - PubMed
-
- Lin SC, Bergles DE. Physiological characteristics of NG2-expressing glial cells. J Neurocytol. 2002;31:537–549. - PubMed
-
- Eroglu C, Barres BA, Stevens B. In: Structural and Functional Organization of the Synapse. Hell JW, Ehlers MD, editors. Springer; 2008. pp. 683–714.
-
- Feng Z, Ko CP. Neuronal glia interactions at the vertebrate neuromuscular junction. Curr Opin Pharmacol. 2007;7:316–324. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

