Methods and options for the heterologous production of complex natural products
- PMID: 21060956
- PMCID: PMC9896020
- DOI: 10.1039/c0np00037j
Methods and options for the heterologous production of complex natural products
Abstract
This review will detail the motivations, experimental approaches, and growing list of successful cases associated with the heterologous production of complex natural products.
Figures
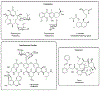


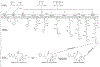
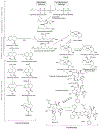

Similar articles
-
Natural Products: Potential Source of DPP-IV Inhibitors.Curr Protein Pept Sci. 2019;20(12):1218-1225. doi: 10.2174/1389203720666190502154129. Curr Protein Pept Sci. 2019. PMID: 31057098 Review.
-
A protocol for HPLC-based activity profiling for natural products with activities against tropical parasites.Nat Prod Commun. 2009 Oct;4(10):1377-81. Nat Prod Commun. 2009. PMID: 19911575
-
A Recent Look into Natural Products that have Potential to Inhibit Cholinesterases and Monoamine Oxidase B: Update for 2010-2019.Comb Chem High Throughput Screen. 2020;23(9):862-876. doi: 10.2174/1386207323666200127145246. Comb Chem High Throughput Screen. 2020. PMID: 31985374 Review.
-
Crystalline-Sponge-Based Structural Analysis of Crude Natural Product Extracts.Angew Chem Int Ed Engl. 2018 Mar 26;57(14):3671-3675. doi: 10.1002/anie.201713219. Epub 2018 Mar 5. Angew Chem Int Ed Engl. 2018. PMID: 29417714
-
Bioactive natural products: function first.Nat Chem. 2011 Jul 22;3(8):575-6. doi: 10.1038/nchem.1105. Nat Chem. 2011. PMID: 21778972 No abstract available.
Cited by
-
Comparative mass spectrometry-based metabolomics strategies for the investigation of microbial secondary metabolites.Nat Prod Rep. 2017 Jan 4;34(1):6-24. doi: 10.1039/c6np00048g. Nat Prod Rep. 2017. PMID: 27604382 Free PMC article. Review.
-
Heterologous Metabolic Pathways: Strategies for Optimal Expression in Eukaryotic Hosts.Acta Naturae. 2020 Apr-Jun;12(2):28-39. doi: 10.32607/actanaturae.10966. Acta Naturae. 2020. PMID: 32742725 Free PMC article.
-
Discovery of acylphloroglucinol-based meroterpenoid enantiomers as KSHV inhibitors from Hypericum japonicum.RSC Adv. 2018 Jul 2;8(43):24101-24109. doi: 10.1039/c8ra04073g. eCollection 2018 Jul 2. RSC Adv. 2018. PMID: 35539193 Free PMC article.
-
Cell-free synthetic biology for in vitro biosynthesis of pharmaceutical natural products.Synth Syst Biotechnol. 2018 Feb 17;3(2):83-89. doi: 10.1016/j.synbio.2018.02.002. eCollection 2018 Jun. Synth Syst Biotechnol. 2018. PMID: 29900420 Free PMC article. Review.
-
Reconstruction of Secondary Metabolic Pathway to Synthesize Novel Metabolite in Saccharopolyspora erythraea.Front Bioeng Biotechnol. 2021 Jul 2;9:628569. doi: 10.3389/fbioe.2021.628569. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34277577 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

