Actin depolymerizing factors cofilin1 and destrin are required for ureteric bud branching morphogenesis
- PMID: 21060807
- PMCID: PMC2965756
- DOI: 10.1371/journal.pgen.1001176
Actin depolymerizing factors cofilin1 and destrin are required for ureteric bud branching morphogenesis
Abstract
The actin depolymerizing factors (ADFs) play important roles in several cellular processes that require cytoskeletal rearrangements, such as cell migration, but little is known about the in vivo functions of ADFs in developmental events like branching morphogenesis. While the molecular control of ureteric bud (UB) branching during kidney development has been extensively studied, the detailed cellular events underlying this process remain poorly understood. To gain insight into the role of actin cytoskeletal dynamics during renal branching morphogenesis, we studied the functional requirements for the closely related ADFs cofilin1 (Cfl1) and destrin (Dstn) during mouse development. Either deletion of Cfl1 in UB epithelium or an inactivating mutation in Dstn has no effect on renal morphogenesis, but simultaneous lack of both genes arrests branching morphogenesis at an early stage, revealing considerable functional overlap between cofilin1 and destrin. Lack of Cfl1 and Dstn in the UB causes accumulation of filamentous actin, disruption of normal epithelial organization, and defects in cell migration. Animals with less severe combinations of mutant Cfl1 and Dstn alleles, which retain one wild-type Cfl1 or Dstn allele, display abnormalities including ureter duplication, renal hypoplasia, and abnormal kidney shape. The results indicate that ADF activity, provided by either cofilin1 or destrin, is essential in UB epithelial cells for normal growth and branching.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
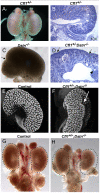
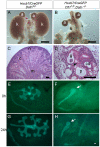
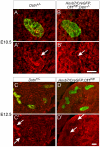
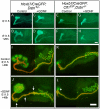
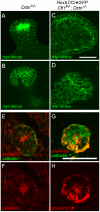
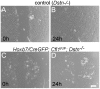
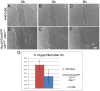
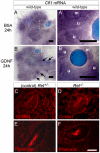
Similar articles
-
Reduction of BMP4 activity by gremlin 1 enables ureteric bud outgrowth and GDNF/WNT11 feedback signalling during kidney branching morphogenesis.Development. 2007 Jul;134(13):2397-405. doi: 10.1242/dev.02861. Epub 2007 May 23. Development. 2007. PMID: 17522159
-
Serum response factor: positive and negative regulation of an epithelial gene expression network in the destrin mutant cornea.Physiol Genomics. 2014 Apr 15;46(8):277-89. doi: 10.1152/physiolgenomics.00126.2013. Epub 2014 Feb 18. Physiol Genomics. 2014. PMID: 24550211 Free PMC article.
-
Angiotensin II AT2 receptor regulates ureteric bud morphogenesis.Am J Physiol Renal Physiol. 2010 Mar;298(3):F807-17. doi: 10.1152/ajprenal.00147.2009. Epub 2009 Dec 23. Am J Physiol Renal Physiol. 2010. PMID: 20032120 Free PMC article.
-
Semaphorins in kidney development and disease: modulators of ureteric bud branching, vascular morphogenesis, and podocyte-endothelial crosstalk.Pediatr Nephrol. 2011 Sep;26(9):1407-12. doi: 10.1007/s00467-011-1769-1. Epub 2011 Feb 20. Pediatr Nephrol. 2011. PMID: 21336944 Free PMC article. Review.
-
Renin-angiotensin system-growth factor cross-talk: a novel mechanism for ureteric bud morphogenesis.Pediatr Nephrol. 2009 Jun;24(6):1113-20. doi: 10.1007/s00467-008-1021-9. Epub 2008 Oct 29. Pediatr Nephrol. 2009. PMID: 18958502 Free PMC article. Review.
Cited by
-
Embryonic Kidney Development, Stem Cells and the Origin of Wilms Tumor.Genes (Basel). 2021 Feb 23;12(2):318. doi: 10.3390/genes12020318. Genes (Basel). 2021. PMID: 33672414 Free PMC article. Review.
-
Receptor tyrosine kinases in kidney development.J Signal Transduct. 2011;2011:869281. doi: 10.1155/2011/869281. Epub 2011 Mar 3. J Signal Transduct. 2011. PMID: 21637383 Free PMC article.
-
Sox9 plays multiple roles in the lung epithelium during branching morphogenesis.Proc Natl Acad Sci U S A. 2013 Nov 19;110(47):E4456-64. doi: 10.1073/pnas.1311847110. Epub 2013 Nov 4. Proc Natl Acad Sci U S A. 2013. PMID: 24191021 Free PMC article.
-
Changes in ADF/destrin expression in the development of hair cells following Atoh1-induced ectopic regeneration.Exp Ther Med. 2013 Jul;6(1):177-183. doi: 10.3892/etm.2013.1089. Epub 2013 Apr 29. Exp Ther Med. 2013. PMID: 23935742 Free PMC article.
-
Coordination of Cellular Dynamics Contributes to Tooth Epithelium Deformations.PLoS One. 2016 Sep 2;11(9):e0161336. doi: 10.1371/journal.pone.0161336. eCollection 2016. PLoS One. 2016. PMID: 27588418 Free PMC article.
References
-
- Van Troys M, Huyck L, Leyman S, Dhaese S, Vandekerkhove J, et al. Ins and outs of ADF/cofilin activity and regulation. Eur J Cell Biol. 2008;87:649–667. - PubMed
-
- Insall RH, Machesky LM. Actin dynamics at the leading edge: from simple machinery to complex networks. Dev Cell. 2009;17:310–322. - PubMed
-
- Lenart P, Bacher CP, Daigle N, Hand AR, Eils R, et al. A contractile nuclear actin network drives chromosome congression in oocytes. Nature. 2005;436:812–818. - PubMed
-
- Gurniak CB, Perlas E, Witke W. The actin depolymerizing factor n-cofilin is essential for neural tube morphogenesis and neural crest cell migration. Dev Biol. 2005;278:231–241. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

