Regulators of the proteasome pathway, Uch37 and Rpn13, play distinct roles in mouse development
- PMID: 21048919
- PMCID: PMC2965108
- DOI: 10.1371/journal.pone.0013654
Regulators of the proteasome pathway, Uch37 and Rpn13, play distinct roles in mouse development
Abstract
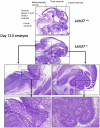
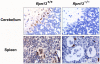
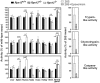
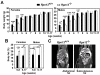
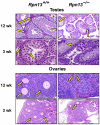
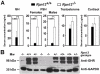
Rpn13 is a novel mammalian proteasomal receptor that has recently been identified as an amplification target in ovarian cancer. It can interact with ubiquitin and activate the deubiquitinating enzyme Uch37 at the 26S proteasome. Since neither Rpn13 nor Uch37 is an integral proteasomal subunit, we explored whether either protein is essential for mammalian development and survival. Deletion of Uch37 resulted in prenatal lethality in mice associated with severe defect in embryonic brain development. In contrast, the majority of Rpn13-deficient mice survived to adulthood, although they were smaller at birth and fewer in number than wild-type littermates. Absence of Rpn13 produced tissue-specific effects on proteasomal function: increased proteasome activity in adrenal gland and lymphoid organs, and decreased activity in testes and brain. Adult Rpn13(-/-) mice reached normal body weight but had increased body fat content and were infertile due to defective gametogenesis. Additionally, Rpn13(-/-) mice showed increased T-cell numbers, resembling growth hormone-mediated effects. Indeed, serum growth hormone and follicular stimulating hormone levels were significantly increased in Rpn13(-/-) mice, while growth hormone receptor expression was reduced in the testes. In conclusion, this is the first report characterizing the physiological roles of Uch37 and Rpn13 in murine development and implicating a non-ATPase proteasomal protein, Rpn13, in the process of gametogenesis.
Conflict of interest statement
Figures


Similar articles
-
Regulation of NF-kappaB activity and inducible nitric oxide synthase by regulatory particle non-ATPase subunit 13 (Rpn13).Proc Natl Acad Sci U S A. 2010 Aug 3;107(31):13854-9. doi: 10.1073/pnas.0913495107. Epub 2010 Jul 15. Proc Natl Acad Sci U S A. 2010. PMID: 20634424 Free PMC article.
-
Proteasomal deubiquitinase UCH37 inhibits degradation of β-catenin and promotes cell proliferation and motility.Acta Biochim Biophys Sin (Shanghai). 2019 Mar 1;51(3):277-284. doi: 10.1093/abbs/gmy176. Acta Biochim Biophys Sin (Shanghai). 2019. PMID: 30726867
-
Ubiquitin docking at the proteasome through a novel pleckstrin-homology domain interaction.Nature. 2008 May 22;453(7194):548-52. doi: 10.1038/nature06924. Nature. 2008. PMID: 18497827 Free PMC article.
-
Power and promise of ubiquitin carboxyl-terminal hydrolase 37 as a target of cancer therapy.Asian Pac J Cancer Prev. 2013;14(4):2173-9. doi: 10.7314/apjcp.2013.14.4.2173. Asian Pac J Cancer Prev. 2013. PMID: 23725108 Review.
-
Meddling with Fate: The Proteasomal Deubiquitinating Enzymes.J Mol Biol. 2017 Nov 10;429(22):3525-3545. doi: 10.1016/j.jmb.2017.09.015. Epub 2017 Oct 5. J Mol Biol. 2017. PMID: 28988953 Free PMC article. Review.
Cited by
-
Inactive USP14 and inactive UCHL5 cause accumulation of distinct ubiquitinated proteins in mammalian cells.PLoS One. 2019 Nov 8;14(11):e0225145. doi: 10.1371/journal.pone.0225145. eCollection 2019. PLoS One. 2019. PMID: 31703099 Free PMC article.
-
Deubiquitinating enzymes (DUBs): decipher underlying basis of neurodegenerative diseases.Mol Psychiatry. 2022 Jan;27(1):259-268. doi: 10.1038/s41380-021-01233-8. Epub 2021 Jul 20. Mol Psychiatry. 2022. PMID: 34285347 Review.
-
A bis-benzylidine piperidone targeting proteasome ubiquitin receptor RPN13/ADRM1 as a therapy for cancer.Cancer Cell. 2013 Dec 9;24(6):791-805. doi: 10.1016/j.ccr.2013.11.001. Cancer Cell. 2013. PMID: 24332045 Free PMC article.
-
Polyubiquitinated proteins, proteasome, and glycogen characterize the particle-rich cytoplasmic structure (PaCS) of neoplastic and fetal cells.Histochem Cell Biol. 2014 May;141(5):483-97. doi: 10.1007/s00418-014-1202-5. Epub 2014 Mar 1. Histochem Cell Biol. 2014. PMID: 24577783
-
Proteasome-Bound UCH37/UCHL5 Debranches Ubiquitin Chains to Promote Degradation.Mol Cell. 2020 Dec 3;80(5):796-809.e9. doi: 10.1016/j.molcel.2020.10.017. Epub 2020 Nov 5. Mol Cell. 2020. PMID: 33156996 Free PMC article.
References
-
- Shimada S, Ogawa M, Schlom J, Greiner JW. Comparison of the interferon-gamma-mediated regulation of tumor-associated antigens expressed by human gastric carcinoma cells. In Vivo. 1993;7:1–8. - PubMed
-
- Simins AB, Weighardt H, Weidner KM, Weidle UH, Holzmann B. Functional cloning of ARM-1, an adhesion-regulating molecule upregulated in metastatic tumor cells. Clin Exp Metastasis. 1999;17:641–648. - PubMed
-
- Shimada S, Ogawa M, Takahashi M, Schlom J, Greiner JW. Molecular cloning and characterization of the complementary DNA of an M(r) 110,000 antigen expressed by human gastric carcinoma cells and upregulated by gamma-interferon. Cancer Res. 1994;54:3831–3836. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

