Imaging lung manifestations of HIV/AIDS
- PMID: 20981180
- PMCID: PMC2954374
- DOI: 10.4103/1817-1737.69106
Imaging lung manifestations of HIV/AIDS
Abstract
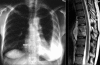
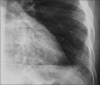
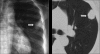
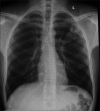
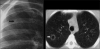
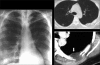
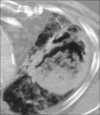
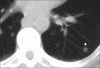
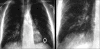
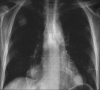
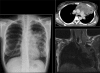
Advances in our understanding of human immunodeficiency virus (HIV) infection have led to improved care and incremental increases in survival. However, the pulmonary manifestations of HIV/acquired immunodeficiency syndrome (AIDS) remain a major cause of morbidity and mortality. Respiratory complaints are not infrequent in patients who are HIV positive. The great majority of lung complications of HIV/AIDS are of infectious etiology but neoplasm, interstitial pneumonias, Kaposi sarcoma and lymphomas add significantly to patient morbidity and mortality. Imaging plays a vital role in the diagnosis and management of lung of complications associated with HIV. Accurate diagnosis is based on an understanding of the pathogenesis of the processes involved and their imaging findings. Imaging also plays an important role in selection of the most appropriate site for tissue sampling, staging of disease and follow-ups. We present images of lung manifestations of HIV/AIDS, describing the salient features and the differential diagnosis.
Keywords: HIV/AIDS; imaging lung; mediastinal manifestations.
Conflict of interest statement
Figures
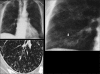
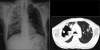
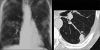
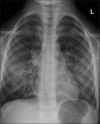
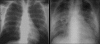
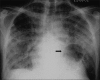
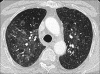
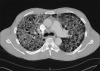
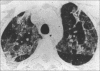
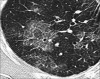
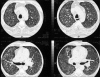
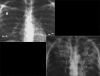
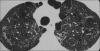
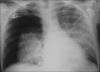

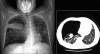
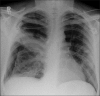
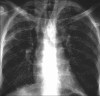
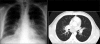
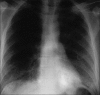
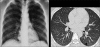
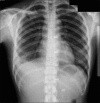
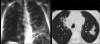
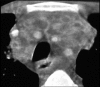
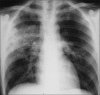
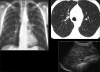
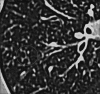
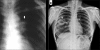
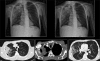

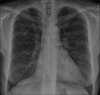
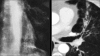
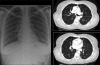
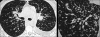

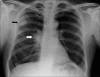
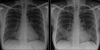
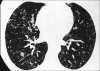
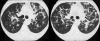
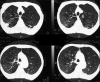
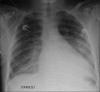
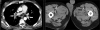

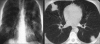
Similar articles
-
HIV-related Malignancies and Mimics: Imaging Findings and Management.Radiographics. 2018 Nov-Dec;38(7):2051-2068. doi: 10.1148/rg.2018180149. Epub 2018 Oct 19. Radiographics. 2018. PMID: 30339518 Review.
-
Human immunodeficiency virus infection in childhood.Ann Trop Paediatr. 1988 Mar;8(1):1-17. doi: 10.1080/02724936.1988.11748530. Ann Trop Paediatr. 1988. PMID: 2456715 Review.
-
Is HIV involved in the pathogenesis of non-infectious pulmonary complications in infected patients?Curr HIV Res. 2003 Oct;1(4):385-93. doi: 10.2174/1570162033485177. Curr HIV Res. 2003. PMID: 15049425 Review.
-
Tuberculosis.In: Holmes KK, Bertozzi S, Bloom BR, Jha P, editors. Major Infectious Diseases. 3rd edition. Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2017 Nov 3. Chapter 11. In: Holmes KK, Bertozzi S, Bloom BR, Jha P, editors. Major Infectious Diseases. 3rd edition. Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2017 Nov 3. Chapter 11. PMID: 30212088 Free Books & Documents. Review.
-
Noninfectious pulmonary complications of HIV/AIDS.Curr Opin Pulm Med. 2005 May;11(3):208-12. doi: 10.1097/01.mcp.0000159833.28271.00. Curr Opin Pulm Med. 2005. PMID: 15818181 Review.
Cited by
-
Unusual imaging findings of SARS-CoV-2 in HIV-positive patient : A case report.Clin Case Rep. 2021 May 15;9(5):e04004. doi: 10.1002/ccr3.4004. eCollection 2021 May. Clin Case Rep. 2021. PMID: 34026131 Free PMC article.
-
Human immunodeficiency virus-associated lung malignancies.Clin Chest Med. 2013 Jun;34(2):255-72. doi: 10.1016/j.ccm.2013.01.008. Epub 2013 Apr 8. Clin Chest Med. 2013. PMID: 23702175 Free PMC article. Review.
-
Chest X-ray Features of HIV-Associated Pneumocystis Pneumonia (PCP) in Adults: A Systematic Review and Meta-analysis.Open Forum Infect Dis. 2024 Mar 18;11(4):ofae146. doi: 10.1093/ofid/ofae146. eCollection 2024 Apr. Open Forum Infect Dis. 2024. PMID: 38628951 Free PMC article.
-
Spontaneous pneumothorax and COVID-19: Precipitants to a complex HIV-AIDS diagnosis.Radiol Case Rep. 2023 Mar;18(3):1197-1200. doi: 10.1016/j.radcr.2022.12.010. Epub 2023 Jan 11. Radiol Case Rep. 2023. PMID: 36643600 Free PMC article.
-
Characteristics and outcomes of patients admitted to a tertiary academic hospital in Pretoria with HIV and severe pneumonia: a retrospective cohort study.BMC Infect Dis. 2022 Jun 15;22(1):548. doi: 10.1186/s12879-022-07522-z. BMC Infect Dis. 2022. PMID: 35705920 Free PMC article.
References
-
- The latest statistics on the world epidemic of HIV and AIDS were published by UNAIDS/WHO in July 2008, and refer to the end of 2007
-
- Beck JM, Rosen MJ, Peavy HH. Pulmonary complications of HIV infection: Report of the Fourth NHLBI Workshop. Am J Respir Crit Care Med. 2001;164:2120–6. - PubMed
-
- Rosen MJ. Overview of pulmonary complications. Clin Chest Med. 1996;17:621–31. - PubMed
-
- Poirier CD, Inhaber N, Lalonde RG, Ernst P. Prevalence of bronchial hyperrsponsiveness among HIV-infected men. Am J Respir Crit Care Med. 2001;164:542–5. - PubMed

