Amyloid beta-protein dimers rapidly form stable synaptotoxic protofibrils
- PMID: 20980598
- PMCID: PMC2987723
- DOI: 10.1523/JNEUROSCI.3537-10.2010
Amyloid beta-protein dimers rapidly form stable synaptotoxic protofibrils
Abstract
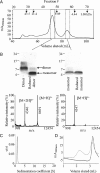
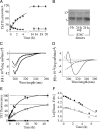
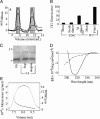
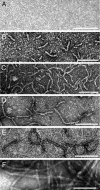
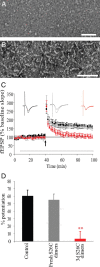
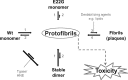
Nonfibrillar, water-soluble low-molecular weight assemblies of the amyloid β-protein (Aβ) are believed to play an important role in Alzheimer's disease (AD). Aqueous extracts of human brain contain Aβ assemblies that migrate on SDS-polyacrylamide gels and elute from size exclusion as dimers (∼8 kDa) and can block long-term potentiation and impair memory consolidation in the rat. Such species are detected specifically and sensitively in extracts of Alzheimer brain suggesting that SDS-stable dimers may be the basic building blocks of AD-associated synaptotoxic assemblies. Consequently, understanding the structure and properties of Aβ dimers is of great interest. In the absence of sufficient brain-derived dimer to facilitate biophysical analysis, we generated synthetic dimers designed to mimic the natural species. For this, Aβ(1-40) containing cysteine in place of serine 26 was used to produce disulphide cross-linked dimer, (AβS26C)2. Such dimers had no detectable secondary structure, produced an analytical ultracentrifugation profile consistent for an ∼8.6 kDa protein, and had no effect on hippocampal long-term potentiation (LTP). However, (AβS26C)2 aggregated more rapidly than either AβS26C or wild-type monomers and formed parastable β-sheet rich, thioflavin T-positive, protofibril-like assemblies. Whereas wild-type Aβ aggregated to form typical amyloid fibrils, the protofibril-like structures formed by (AβS26C)2 persisted for prolonged periods and potently inhibited LTP in mouse hippocampus. These data support the idea that Aβ dimers may stabilize the formation of fibril intermediates by a process distinct from that available to Aβ monomer and that higher molecular weight prefibrillar assemblies are the proximate mediators of Aβ toxicity.
Figures
Similar articles
-
A monoclonal antibody against synthetic Aβ dimer assemblies neutralizes brain-derived synaptic plasticity-disrupting Aβ.J Neurochem. 2011 Oct;119(1):189-201. doi: 10.1111/j.1471-4159.2011.07389.x. Epub 2011 Aug 22. J Neurochem. 2011. PMID: 21781116 Free PMC article.
-
Aβ dimers differ from monomers in structural propensity, aggregation paths and population of synaptotoxic assemblies.Biochem J. 2014 Aug 1;461(3):413-26. doi: 10.1042/BJ20140219. Biochem J. 2014. PMID: 24785004
-
Human anti-Aβ IgGs target conformational epitopes on synthetic dimer assemblies and the AD brain-derived peptide.PLoS One. 2012;7(11):e50317. doi: 10.1371/journal.pone.0050317. Epub 2012 Nov 27. PLoS One. 2012. PMID: 23209707 Free PMC article.
-
Is covalently crosslinked Abeta responsible for synaptotoxicity in Alzheimer's disease?Curr Alzheimer Res. 2008 Dec;5(6):533-9. doi: 10.2174/156720508786898433. Curr Alzheimer Res. 2008. PMID: 19075579 Review.
-
Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior.Behav Brain Res. 2008 Sep 1;192(1):106-13. doi: 10.1016/j.bbr.2008.02.016. Epub 2008 Feb 17. Behav Brain Res. 2008. PMID: 18359102 Free PMC article. Review.
Cited by
-
Multivariate analyses of amyloid-beta oligomer populations indicate a connection between pore formation and cytotoxicity.PLoS One. 2012;7(10):e47261. doi: 10.1371/journal.pone.0047261. Epub 2012 Oct 15. PLoS One. 2012. PMID: 23077580 Free PMC article.
-
Short-term fish oil supplementation applied in presymptomatic stage of Alzheimer's disease enhances microglial/macrophage barrier and prevents neuritic dystrophy in parietal cortex of 5xFAD mouse model.PLoS One. 2019 May 16;14(5):e0216726. doi: 10.1371/journal.pone.0216726. eCollection 2019. PLoS One. 2019. PMID: 31095617 Free PMC article.
-
An alternative structural isoform in amyloid-like aggregates formed from thermally denatured human γD-crystallin.Protein Sci. 2014 Mar;23(3):321-31. doi: 10.1002/pro.2422. Epub 2014 Feb 4. Protein Sci. 2014. PMID: 24415662 Free PMC article.
-
A Path Toward Precision Medicine for Neuroinflammatory Mechanisms in Alzheimer's Disease.Front Immunol. 2020 Mar 31;11:456. doi: 10.3389/fimmu.2020.00456. eCollection 2020. Front Immunol. 2020. PMID: 32296418 Free PMC article. Review.
-
Amyloid-β(1-42) protofibrils stimulate a quantum of secreted IL-1β despite significant intracellular IL-1β accumulation in microglia.Biochim Biophys Acta. 2014 Nov;1842(11):2276-85. doi: 10.1016/j.bbadis.2014.08.001. Epub 2014 Aug 11. Biochim Biophys Acta. 2014. PMID: 25125050 Free PMC article.
References
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. - PubMed
-
- Harper JD, Lansbury PT., Jr Models of amyloid seeding in Alzheimer's disease and scrapie: mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins. Annu Rev Biochem. 1997;66:385–407. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
