Autocrine interferon priming in macrophages but not dendritic cells results in enhanced cytokine and chemokine production after coronavirus infection
- PMID: 20978536
- PMCID: PMC2957079
- DOI: 10.1128/mBio.00219-10
Autocrine interferon priming in macrophages but not dendritic cells results in enhanced cytokine and chemokine production after coronavirus infection
Abstract
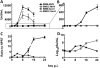
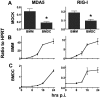
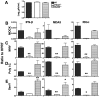
Coronaviruses efficiently inhibit interferon (IFN) induction in nonhematopoietic cells and conventional dendritic cells (cDC). However, IFN is produced in infected macrophages, microglia, and plasmacytoid dendritic cells (pDC). To begin to understand why IFN is produced in infected macrophages, we infected bone marrow-derived macrophages (BMM) and as a control, bone marrow-derived DC (BMDC) with the coronavirus mouse hepatitis virus (MHV). As expected, BMM but not BMDC expressed type I IFN. IFN production in infected BMM was nearly completely dependent on signaling through the alpha/beta interferon (IFN-α/β) receptor (IFNAR). Several IFN-dependent cytokines and chemokines showed the same expression pattern, with enhanced production in BMM compared to BMDC and dependence upon signaling through the IFNAR. Exogenous IFN enhanced IFN-dependent gene expression in BMM at early times after infection and in BMDC at all times after infection but did not stimulate expression of molecules that signal through myeloid differentiation factor 88 (MyD88), such as tumor necrosis factor (TNF). Collectively, our results show that IFN is produced at early times postinfection (p.i.) in MHV-infected BMM, but not in BMDC, and primes expression of IFN and IFN-responsive genes. Further, our results also show that BMM are generally more responsive to MHV infection, since MyD88-dependent pathways are also activated to a greater extent in these cells than in BMDC.
Figures

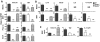
Similar articles
-
Type I IFN-mediated protection of macrophages and dendritic cells secures control of murine coronavirus infection.J Immunol. 2009 Jan 15;182(2):1099-106. doi: 10.4049/jimmunol.182.2.1099. J Immunol. 2009. PMID: 19124753
-
Murine coronavirus mouse hepatitis virus is recognized by MDA5 and induces type I interferon in brain macrophages/microglia.J Virol. 2008 Oct;82(20):9829-38. doi: 10.1128/JVI.01199-08. Epub 2008 Jul 30. J Virol. 2008. PMID: 18667505 Free PMC article.
-
Neuronal Ablation of Alpha/Beta Interferon (IFN-α/β) Signaling Exacerbates Central Nervous System Viral Dissemination and Impairs IFN-γ Responsiveness in Microglia/Macrophages.J Virol. 2020 Sep 29;94(20):e00422-20. doi: 10.1128/JVI.00422-20. Print 2020 Sep 29. J Virol. 2020. PMID: 32796063 Free PMC article.
-
Cell-type-specific type I interferon antagonism influences organ tropism of murine coronavirus.J Virol. 2011 Oct;85(19):10058-68. doi: 10.1128/JVI.05075-11. Epub 2011 Jul 13. J Virol. 2011. PMID: 21752905 Free PMC article.
-
Coronavirus infection of polarized epithelial cells.Trends Microbiol. 1995 Dec;3(12):486-90. doi: 10.1016/s0966-842x(00)89018-6. Trends Microbiol. 1995. PMID: 8800844 Free PMC article. Review.
Cited by
-
N7-Methylation of the Coronavirus RNA Cap Is Required for Maximal Virulence by Preventing Innate Immune Recognition.mBio. 2022 Feb 22;13(1):e0366221. doi: 10.1128/mbio.03662-21. Epub 2022 Jan 25. mBio. 2022. PMID: 35073761 Free PMC article.
-
Protective role of Toll-like Receptor 3-induced type I interferon in murine coronavirus infection of macrophages.Viruses. 2012 May;4(5):901-23. doi: 10.3390/v4050901. Epub 2012 May 24. Viruses. 2012. PMID: 22754655 Free PMC article.
-
Ribose 2'-O-methylation provides a molecular signature for the distinction of self and non-self mRNA dependent on the RNA sensor Mda5.Nat Immunol. 2011 Feb;12(2):137-43. doi: 10.1038/ni.1979. Epub 2011 Jan 9. Nat Immunol. 2011. PMID: 21217758 Free PMC article.
-
IFN-I response timing relative to virus replication determines MERS coronavirus infection outcomes.J Clin Invest. 2019 Jul 29;129(9):3625-3639. doi: 10.1172/JCI126363. eCollection 2019 Jul 29. J Clin Invest. 2019. PMID: 31355779 Free PMC article.
-
The N-Terminal Region of Middle East Respiratory Syndrome Coronavirus Accessory Protein 8b Is Essential for Enhanced Virulence of an Attenuated Murine Coronavirus.J Virol. 2022 Feb 9;96(3):e0184221. doi: 10.1128/JVI.01842-21. Epub 2021 Nov 24. J Virol. 2022. PMID: 34817197 Free PMC article.
References
-
- Garcia-Sastre A., Biron C. A. 2006. Type 1 interferons and the virus-host relationship: a lesson in detente. Science 312:879–882 - PubMed
-
- Hengel H., Koszinowski U. H., Conzelmann K. K. 2005. Viruses know it all: new insights into IFN networks. Trends Immunol. 26:396–401 - PubMed
-
- Perry A. K., Chen G., Zheng D., Tang H., Cheng G. 2005. The host type I interferon response to viral and bacterial infections. Cell Res. 15:407–422 - PubMed
-
- Sarkar S. N., Sen G. C. 2004. Novel functions of proteins encoded by viral stress-inducible genes. Pharmacol. Ther. 103:245–259 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

