Conserved molecular mechanisms underlying homeostasis of the Golgi complex
- PMID: 20976261
- PMCID: PMC2952910
- DOI: 10.1155/2010/758230
Conserved molecular mechanisms underlying homeostasis of the Golgi complex
Abstract
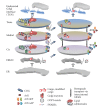
The Golgi complex performs a central function in the secretory pathway in the sorting and sequential processing of a large number of proteins destined for other endomembrane organelles, the plasma membrane, or secretion from the cell, in addition to lipid metabolism and signaling. The Golgi apparatus can be regarded as a self-organizing system that maintains a relatively stable morphofunctional organization in the face of an enormous flux of lipids and proteins. A large number of the molecular players that operate in these processes have been identified, their functions and interactions defined, but there is still debate about many aspects that regulate protein trafficking and, in particular, the maintenance of these highly dynamic structures and processes. Here, we consider how an evolutionarily conserved underlying mechanism based on retrograde trafficking that uses lipids, COPI, SNAREs, and tethers could maintain such a homeodynamic system.
Figures

Similar articles
-
TRP Channel Trafficking.In: Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 23. In: Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 23. PMID: 21204515 Free Books & Documents. Review.
-
Creating Knockouts of Conserved Oligomeric Golgi Complex Subunits Using CRISPR-Mediated Gene Editing Paired with a Selection Strategy Based on Glycosylation Defects Associated with Impaired COG Complex Function.Methods Mol Biol. 2016;1496:145-61. doi: 10.1007/978-1-4939-6463-5_12. Methods Mol Biol. 2016. PMID: 27632008 Free PMC article.
-
Building a secretory apparatus: role of ARF1/COPI in Golgi biogenesis and maintenance.Histochem Cell Biol. 1998 May-Jun;109(5-6):449-62. doi: 10.1007/s004180050247. Histochem Cell Biol. 1998. PMID: 9681627 Review.
-
The COG complex, Rab6 and COPI define a novel Golgi retrograde trafficking pathway that is exploited by SubAB toxin.Traffic. 2009 Oct;10(10):1502-17. doi: 10.1111/j.1600-0854.2009.00965.x. Epub 2009 Jul 21. Traffic. 2009. PMID: 19678899 Free PMC article.
-
The Sec34/Sec35p complex, a Ypt1p effector required for retrograde intra-Golgi trafficking, interacts with Golgi SNAREs and COPI vesicle coat proteins.J Cell Biol. 2002 May 13;157(4):631-43. doi: 10.1083/jcb.200111081. Epub 2002 May 13. J Cell Biol. 2002. PMID: 12011112 Free PMC article.
Cited by
-
Stacks off tracks: a role for the golgin AtCASP in plant endoplasmic reticulum-Golgi apparatus tethering.J Exp Bot. 2017 Jun 15;68(13):3339-3350. doi: 10.1093/jxb/erx167. J Exp Bot. 2017. PMID: 28605454 Free PMC article.
-
The Role of the Transmembrane RING Finger Proteins in Cellular and Organelle Function.Membranes (Basel). 2011 Dec 9;1(4):354-93. doi: 10.3390/membranes1040354. Membranes (Basel). 2011. PMID: 24957874 Free PMC article.
-
Isoform-specific tethering links the Golgi ribbon to maintain compartmentalization.Mol Biol Cell. 2014 Jan;25(1):133-44. doi: 10.1091/mbc.E13-07-0395. Epub 2013 Nov 13. Mol Biol Cell. 2014. PMID: 24227884 Free PMC article.
-
The endoplasmic reticulum-resident chaperone heat shock protein 47 protects the Golgi apparatus from the effects of O-glycosylation inhibition.PLoS One. 2013 Jul 29;8(7):e69732. doi: 10.1371/journal.pone.0069732. Print 2013. PLoS One. 2013. PMID: 23922785 Free PMC article.
-
Quantitative analysis of intra-Golgi transport shows intercisternal exchange for all cargo.Proc Natl Acad Sci U S A. 2013 Sep 24;110(39):15692-7. doi: 10.1073/pnas.1303358110. Epub 2013 Sep 9. Proc Natl Acad Sci U S A. 2013. PMID: 24019488 Free PMC article.
References
-
- Latijnhouwers M, Hawes C, Carvalho C. Holding it all together? Candidate proteins for the plant Golgi matrix. Current Opinion in Plant Biology. 2005;8(6):632–639. - PubMed
-
- Kondylis V, Rabouille C. The Golgi apparatus: lessons from Drosophila . FEBS Letters. 2009;583(23):3827–3838. - PubMed
-
- Rambourg A, Jackson CL, Clermont Y. Three dimensional configuration of the secretory pathway and segregation of secretion granules in the yeast Saccharomyces cerevisiae. The Journal of Cell Science. 2001;114(12):2231–2239. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

