Inhibition of cellular protein secretion by norwalk virus nonstructural protein p22 requires a mimic of an endoplasmic reticulum export signal
- PMID: 20976190
- PMCID: PMC2956632
- DOI: 10.1371/journal.pone.0013130
Inhibition of cellular protein secretion by norwalk virus nonstructural protein p22 requires a mimic of an endoplasmic reticulum export signal
Abstract
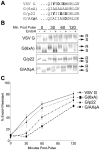
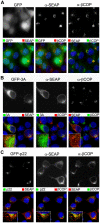
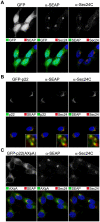
Protein trafficking between the endoplasmic reticulum (ER) and Golgi apparatus is central to cellular homeostasis. ER export signals are utilized by a subset of proteins to rapidly exit the ER by direct uptake into COPII vesicles for transport to the Golgi. Norwalk virus nonstructural protein p22 contains a YXΦESDG motif that mimics a di-acidic ER export signal in both sequence and function. However, unlike normal ER export signals, the ER export signal mimic of p22 is necessary for apparent inhibition of normal COPII vesicle trafficking, which leads to Golgi disassembly and antagonism of Golgi-dependent cellular protein secretion. This is the first reported function for p22. Disassembly of the Golgi apparatus was also observed in cells replicating Norwalk virus, which may contribute to pathogenesis by interfering with cellular processes that are dependent on an intact secretory pathway. These results indicate that the ER export signal mimic is critical to the antagonistic function of p22, shown herein to be a novel antagonist of ER/Golgi trafficking. This unique and well-conserved human norovirus motif is therefore an appealing target for antiviral drug development.
Conflict of interest statement
Figures

Similar articles
-
Secretory pathway antagonism by calicivirus homologues of Norwalk virus nonstructural protein p22 is restricted to noroviruses.Virol J. 2012 Sep 3;9:181. doi: 10.1186/1743-422X-9-181. Virol J. 2012. PMID: 22943503 Free PMC article.
-
Vesicle-mediated export from the ER: COPII coat function and regulation.Biochim Biophys Acta. 2013 Nov;1833(11):2464-72. doi: 10.1016/j.bbamcr.2013.02.003. Epub 2013 Feb 15. Biochim Biophys Acta. 2013. PMID: 23419775 Free PMC article. Review.
-
Effects of foot-and-mouth disease virus nonstructural proteins on the structure and function of the early secretory pathway: 2BC but not 3A blocks endoplasmic reticulum-to-Golgi transport.J Virol. 2005 Apr;79(7):4382-95. doi: 10.1128/JVI.79.7.4382-4395.2005. J Virol. 2005. PMID: 15767438 Free PMC article.
-
Dynamics of COPII vesicles and the Golgi apparatus in cultured Nicotiana tabacum BY-2 cells provides evidence for transient association of Golgi stacks with endoplasmic reticulum exit sites.Plant Cell. 2005 May;17(5):1513-31. doi: 10.1105/tpc.104.026757. Epub 2005 Apr 1. Plant Cell. 2005. PMID: 15805489 Free PMC article.
-
[From endoplasmic reticulum to Golgi apparatus: a secretory pathway controlled by signal molecules].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2013 Jul;42(4):472-7. doi: 10.3785/j.issn.1008-9292.2013.04.017. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2013. PMID: 24022939 Review. Chinese.
Cited by
-
Inherent structural disorder and dimerisation of murine norovirus NS1-2 protein.PLoS One. 2012;7(2):e30534. doi: 10.1371/journal.pone.0030534. Epub 2012 Feb 7. PLoS One. 2012. PMID: 22347381 Free PMC article.
-
Expression of the murine norovirus (MNV) ORF1 polyprotein is sufficient to induce apoptosis in a virus-free cell model.PLoS One. 2014 Mar 5;9(3):e90679. doi: 10.1371/journal.pone.0090679. eCollection 2014. PLoS One. 2014. PMID: 24599381 Free PMC article.
-
Complete genome characterization of human noroviruses allows comparison of minor alleles during acute and chronic infections.Access Microbiol. 2021 Feb 17;3(3):000203. doi: 10.1099/acmi.0.000203. eCollection 2021 Mar. Access Microbiol. 2021. PMID: 34151158 Free PMC article.
-
Subcellular Localization and Functional Characterization of GII.4 Norovirus-Encoded NTPase.J Virol. 2018 Feb 12;92(5):e01824-17. doi: 10.1128/JVI.01824-17. Print 2018 Mar 1. J Virol. 2018. PMID: 29212938 Free PMC article.
-
Norovirus antagonism of B-cell antigen presentation results in impaired control of acute infection.Mucosal Immunol. 2016 Nov;9(6):1559-1570. doi: 10.1038/mi.2016.15. Epub 2016 Mar 23. Mucosal Immunol. 2016. PMID: 27007673 Free PMC article.
References
-
- Scales SJ, Pepperkok R, Kreis TE. Visualization of ER-to-Golgi transport in living cells reveals a sequential mode of action for COPII and COPI. Cell. 1997;90:1137–1148. - PubMed
-
- Schekman R, Barlowe C, Yeung T, Hamamoto S, Hosobuchi D, et al. COPII - A Novel Coat Protein Required for Transport Vesicle Budding from the Endoplasmic-Reticulum. FASEB J. 1994;8:A1379.
-
- Budnik A, Stephens DJ. ER exit sites–localization and control of COPII vesicle formation. FEBS Lett. 2009;583:3796–3803. - PubMed
-
- Tang BL, Wang Y, Ong YS, Hong WJ. COPII and exit from the endoplasmic reticulum. Biochimica et Biophysica Acta-Mol Cell Res. 2005;1744:293–303. - PubMed
-
- Saraste J, Dale HA, Bazzocco S, Marie M. Emerging new roles of the pre-Golgi intermediate compartment in biosynthetic-secretory trafficking. FEBS Lett. 2009;583:3804–3810. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI036211/AI/NIAID NIH HHS/United States
- U54 HD-007495/HD/NICHD NIH HHS/United States
- S10RR024574/RR/NCRR NIH HHS/United States
- P30 DK-56338/DK/NIDDK NIH HHS/United States
- P30 CA125123/CA/NCI NIH HHS/United States
- P30 AI036211/AI/NIAID NIH HHS/United States
- S10 RR024574/RR/NCRR NIH HHS/United States
- P01 AI057788/AI/NIAID NIH HHS/United States
- T32 AI04741/AI/NIAID NIH HHS/United States
- U54 HD007495/HD/NICHD NIH HHS/United States
- P30 CA-125123/CA/NCI NIH HHS/United States
- P30 DK056338/DK/NIDDK NIH HHS/United States

