High-mobility group box 1, oxidative stress, and disease
- PMID: 20969478
- PMCID: PMC3048826
- DOI: 10.1089/ars.2010.3356
High-mobility group box 1, oxidative stress, and disease
Abstract
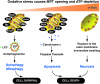
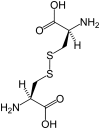
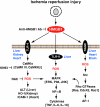
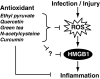
Oxidative stress and associated reactive oxygen species can modify lipids, proteins, carbohydrates, and nucleic acids, and induce the mitochondrial permeability transition, providing a signal leading to the induction of autophagy, apoptosis, and necrosis. High-mobility group box 1 (HMGB1) protein, a chromatin-binding nuclear protein and damage-associated molecular pattern molecule, is integral to oxidative stress and downstream apoptosis or survival. Accumulation of HMGB1 at sites of oxidative DNA damage can lead to repair of the DNA. As a redox-sensitive protein, HMGB1 contains three cysteines (Cys23, 45, and 106). In the setting of oxidative stress, it can form a Cys23-Cys45 disulfide bond; a role for oxidative homo- or heterodimerization through the Cys106 has been suggested for some of its biologic activities. HMGB1 causes activation of nicotinamide adenine dinucleotide phosphate oxidase and increased reactive oxygen species production in neutrophils. Reduced and oxidized HMGB1 have different roles in extracellular signaling and regulation of immune responses, mediated by signaling through the receptor for advanced glycation end products and/or Toll-like receptors. Antioxidants such as ethyl pyruvate, quercetin, green tea, N-acetylcysteine, and curcumin are protective in the setting of experimental infection/sepsis and injury including ischemia-reperfusion, partly through attenuating HMGB1 release and systemic accumulation.
Figures





Similar articles
-
Molecular basis for the redox control of nuclear transport of the structural chromatin protein Hmgb1.Exp Cell Res. 2006 Nov 1;312(18):3526-38. doi: 10.1016/j.yexcr.2006.07.020. Epub 2006 Aug 2. Exp Cell Res. 2006. PMID: 16962095
-
Reactive oxygen species induce Cys106-mediated anti-parallel HMGB1 dimerization that protects against DNA damage.Redox Biol. 2021 Apr;40:101858. doi: 10.1016/j.redox.2021.101858. Epub 2021 Jan 7. Redox Biol. 2021. PMID: 33461096 Free PMC article.
-
Nuclear heat shock protein 72 as a negative regulator of oxidative stress (hydrogen peroxide)-induced HMGB1 cytoplasmic translocation and release.J Immunol. 2007 Jun 1;178(11):7376-84. doi: 10.4049/jimmunol.178.11.7376. J Immunol. 2007. PMID: 17513788 Free PMC article.
-
Redox modulation of HMGB1-related signaling.Antioxid Redox Signal. 2014 Mar 1;20(7):1075-85. doi: 10.1089/ars.2013.5179. Epub 2013 Mar 19. Antioxid Redox Signal. 2014. PMID: 23373897 Free PMC article. Review.
-
Decoding cell death signals in liver inflammation.J Hepatol. 2013 Sep;59(3):583-94. doi: 10.1016/j.jhep.2013.03.033. Epub 2013 Apr 6. J Hepatol. 2013. PMID: 23567086 Review.
Cited by
-
Cardiovascular Disease and miRNAs: Possible Oxidative Stress-Regulating Roles of miRNAs.Antioxidants (Basel). 2024 May 27;13(6):656. doi: 10.3390/antiox13060656. Antioxidants (Basel). 2024. PMID: 38929095 Free PMC article. Review.
-
The Foxo1-YAP-Notch1 axis reprograms STING-mediated innate immunity in NASH progression.Exp Mol Med. 2024 Aug;56(8):1843-1855. doi: 10.1038/s12276-024-01280-5. Epub 2024 Aug 9. Exp Mol Med. 2024. PMID: 39122845 Free PMC article.
-
Oxidative stress-mediated HMGB1 biology.Front Physiol. 2015 Apr 7;6:93. doi: 10.3389/fphys.2015.00093. eCollection 2015. Front Physiol. 2015. PMID: 25904867 Free PMC article. Review.
-
Location is the key to function: HMGB1 in sepsis and trauma-induced inflammation.J Leukoc Biol. 2019 Jul;106(1):161-169. doi: 10.1002/JLB.3MIR1218-497R. Epub 2019 Apr 4. J Leukoc Biol. 2019. PMID: 30946496 Free PMC article. Review.
-
Sickle cell disease increases high mobility group box 1: a novel mechanism of inflammation.Blood. 2014 Dec 18;124(26):3978-81. doi: 10.1182/blood-2014-04-560813. Epub 2014 Oct 22. Blood. 2014. PMID: 25339362 Free PMC article.
References
-
- Abd El-Gawad HM. Khalifa AE. Quercetin, coenzyme Q10, and L-canavanine as protective agents against lipid peroxidation and nitric oxide generation in endotoxin-induced shock in rat brain. Pharmacol Res. 2001;43:257–263. - PubMed
-
- Abraham E. Arcaroli J. Carmody A. Wang H. Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000;165:2950–2954. - PubMed
-
- Agresti A. Lupo R. Bianchi ME. Muller S. HMGB1 interacts differentially with members of the Rel family of transcription factors. Biochem Biophys Res Commun. 2003;302:421–426. - PubMed
-
- Andrassy M. Volz HC. Igwe JC. Funke B. Eichberger SN. Kaya Z. Buss S. Autschbach F. Pleger ST. Lukic IK. Bea F. Hardt SE. Humpert PM. Bianchi ME. Mairbaurl H. Nawroth PP. Remppis A. Katus HA. Bierhaus A. High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation. 2008;117:3216–3226. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

