QM/MM study of dehydro and dihydro β-ionone retinal analogues in squid and bovine rhodopsins: implications for vision in salamander rhodopsin
- PMID: 20964383
- PMCID: PMC2988495
- DOI: 10.1021/ja105050p
QM/MM study of dehydro and dihydro β-ionone retinal analogues in squid and bovine rhodopsins: implications for vision in salamander rhodopsin
Abstract
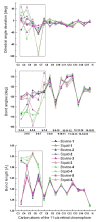
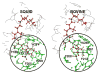
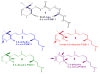
Visual pigment rhodopsin provides a decisive crossing point for interaction between organisms and environment. Naturally occurring visual pigments contain only PSB11 and 3,4-dehydro-PSB11 as chromophores. Therefore, the ability of visual opsin to discriminate between the retinal geometries is investigated by means of QM/MM incorporation of PSB11, 6-s-cis and 6-s-trans forms of 3,4-dehydro-PSB11, and 3,4-dehydro-5,6-dihydro-PSB11 and 5,6-dihydro-PSB11 analogues into squid and bovine rhodopsin environments. The analogue-protein interaction reveals the binding site of squid rhodopsin to be malleable and ductile, while that of bovine rhodopsin is rigid and stiff. On the basis of these studies, a tentative model of the salamander rhodopsin binding site is also proposed.
Figures

Similar articles
-
Rhodopsin regeneration is accelerated via noncovalent 11-cis retinal-opsin complex--a role of retinal binding pocket of opsin.Photochem Photobiol. 2008 Jul-Aug;84(4):985-9. doi: 10.1111/j.1751-1097.2008.00338.x. Epub 2008 Apr 9. Photochem Photobiol. 2008. PMID: 18399914
-
Binding of more than one retinoid to visual opsins.Biophys J. 2010 Oct 6;99(7):2366-73. doi: 10.1016/j.bpj.2010.08.003. Biophys J. 2010. PMID: 20923672 Free PMC article.
-
The C9 methyl group of retinal interacts with glycine-121 in rhodopsin.Proc Natl Acad Sci U S A. 1997 Dec 9;94(25):13442-7. doi: 10.1073/pnas.94.25.13442. Proc Natl Acad Sci U S A. 1997. PMID: 9391044 Free PMC article.
-
Photophysiological functions of visual pigments.Adv Biophys. 1984;17:5-67. doi: 10.1016/0065-227x(84)90024-8. Adv Biophys. 1984. PMID: 6242325 Review.
-
Cone visual pigments.Biochim Biophys Acta. 2014 May;1837(5):664-73. doi: 10.1016/j.bbabio.2013.08.009. Epub 2013 Sep 7. Biochim Biophys Acta. 2014. PMID: 24021171 Review.
Cited by
-
H-bond network around retinal regulates the evolution of ultraviolet and violet vision.ACS Chem Biol. 2011 Aug 19;6(8):775-80. doi: 10.1021/cb200100f. Epub 2011 Jun 14. ACS Chem Biol. 2011. PMID: 21650174 Free PMC article.
-
Why 11-cis-retinal? Why not 7-cis-, 9-cis-, or 13-cis-retinal in the eye?J Am Chem Soc. 2011 Nov 30;133(47):19052-5. doi: 10.1021/ja208789h. Epub 2011 Nov 3. J Am Chem Soc. 2011. PMID: 22026715 Free PMC article.
-
Relationship between Excited State Lifetime and Isomerization Quantum Yield in Animal Rhodopsins: Beyond the One-Dimensional Landau-Zener Model.J Phys Chem Lett. 2018 Jun 21;9(12):3315-3322. doi: 10.1021/acs.jpclett.8b01062. Epub 2018 Jun 6. J Phys Chem Lett. 2018. PMID: 29791163 Free PMC article.
-
Quantum mechanical/molecular mechanical structure, enantioselectivity, and spectroscopy of hydroxyretinals and insights into the evolution of color vision in small white butterflies.J Phys Chem B. 2011 Dec 29;115(51):15380-8. doi: 10.1021/jp208107r. Epub 2011 Dec 6. J Phys Chem B. 2011. PMID: 22087641 Free PMC article.
-
Spectral tuning of ultraviolet cone pigments: an interhelical lock mechanism.J Am Chem Soc. 2013 Dec 26;135(51):19064-7. doi: 10.1021/ja409896y. Epub 2013 Dec 12. J Am Chem Soc. 2013. PMID: 24295328 Free PMC article.
References
-
- Sekharan S, Altun A, Morokuma K. Chem Eur J. 2010;16:1744–1749. - PMC - PubMed
- Sekharan S, Morokuma K. J Phys, Chem Lett. 2010;1:668–672. - PMC - PubMed
- Altun A, Yokoyama S, Morokuma K. J Phys Chem B. 2008;112:16883–168890. - PMC - PubMed
- Altun A, Yokoyama S, Morokuma K. J Phys Chem A. 2009;113:11685–11692. - PMC - PubMed
-
- Dartnall HJ, Lythgoe JN. Vision Res. 1965;5:81–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

