The alphaherpesvirus US3/ORF66 protein kinases direct phosphorylation of the nuclear matrix protein matrin 3
- PMID: 20962082
- PMCID: PMC3014177
- DOI: 10.1128/JVI.01611-10
The alphaherpesvirus US3/ORF66 protein kinases direct phosphorylation of the nuclear matrix protein matrin 3
Abstract
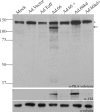
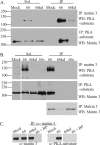
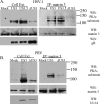
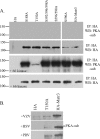
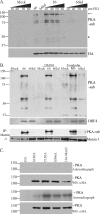
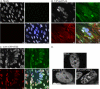
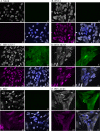
The protein kinase found in the short region of alphaherpesviruses, termed US3 in herpes simplex virus type 1 (HSV-1) and pseudorabies virus (PRV) and ORF66 in varicella-zoster virus (VZV), affects several viral and host cell processes, and its specific targets remain an area of active investigation. Reports suggesting that HSV-1 US3 substrates overlap with those of cellular protein kinase A (PKA) prompted the use of an antibody specific for phosphorylated PKA substrates to identify US3/ORF66 targets. HSV-1, VZV, and PRV induced very different substrate profiles that were US3/ORF66 kinase dependent. The predominant VZV-phosphorylated 125-kDa species was identified as matrin 3, one of the major nuclear matrix proteins. Matrin 3 was also phosphorylated by HSV-1 and PRV in a US3 kinase-dependent manner and by VZV ORF66 kinase at a novel residue (KRRRT150EE). Since VZV-directed T150 phosphorylation was not blocked by PKA inhibitors and was not induced by PKA activation, and since PKA predominantly targeted matrin 3 S188, it was concluded that phosphorylation by VZV was PKA independent. However, purified VZV ORF66 kinase did not phosphorylate matrin 3 in vitro, suggesting that additional cellular factors were required. In VZV-infected cells in the absence of the ORF66 kinase, matrin 3 displayed intranuclear changes, while matrin 3 showed a pronounced cytoplasmic distribution in late-stage cells infected with US3-negative HSV-1 or PRV. This work identifies phosphorylation of the nuclear matrix protein matrin 3 as a new conserved target of this kinase group.
Figures

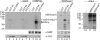
Similar articles
-
Hyperphosphorylation of histone deacetylase 2 by alphaherpesvirus US3 kinases.J Virol. 2010 Oct;84(19):9666-76. doi: 10.1128/JVI.00981-10. Epub 2010 Jul 21. J Virol. 2010. PMID: 20660201 Free PMC article.
-
Varicella-zoster virus open reading frame 66 protein kinase and its relationship to alphaherpesvirus US3 kinases.Curr Top Microbiol Immunol. 2010;342:79-98. doi: 10.1007/82_2009_7. Curr Top Microbiol Immunol. 2010. PMID: 20186610 Free PMC article. Review.
-
Phosphorylation of the varicella-zoster virus (VZV) major transcriptional regulatory protein IE62 by the VZV open reading frame 66 protein kinase.J Virol. 2006 Feb;80(4):1710-23. doi: 10.1128/JVI.80.4.1710-1723.2006. J Virol. 2006. PMID: 16439528 Free PMC article.
-
Nuclear accumulation of IE62, the varicella-zoster virus (VZV) major transcriptional regulatory protein, is inhibited by phosphorylation mediated by the VZV open reading frame 66 protein kinase.J Virol. 2000 Mar;74(5):2265-77. doi: 10.1128/jvi.74.5.2265-2277.2000. J Virol. 2000. PMID: 10666257 Free PMC article.
-
Us3 Protein Kinase Encoded by HSV: The Precise Function and Mechanism on Viral Life Cycle.Adv Exp Med Biol. 2018;1045:45-62. doi: 10.1007/978-981-10-7230-7_3. Adv Exp Med Biol. 2018. PMID: 29896662 Review.
Cited by
-
Matrin 3 in neuromuscular disease: physiology and pathophysiology.JCI Insight. 2021 Jan 11;6(1):e143948. doi: 10.1172/jci.insight.143948. JCI Insight. 2021. PMID: 33427209 Free PMC article. Review.
-
Breach of the nuclear lamina during assembly of herpes simplex viruses.Nucleus. 2011 Jul-Aug;2(4):271-6. doi: 10.4161/nucl.2.4.16334. Epub 2011 Jul 1. Nucleus. 2011. PMID: 21941110 Free PMC article. Review.
-
Suppression of extracellular signal-regulated kinase activity in herpes simplex virus 1-infected cells by the Us3 protein kinase.J Virol. 2012 Aug;86(15):7771-6. doi: 10.1128/JVI.00622-12. Epub 2012 May 16. J Virol. 2012. PMID: 22593153 Free PMC article.
-
Posttranscriptional Regulation of HIV-1 Gene Expression during Replication and Reactivation from Latency by Nuclear Matrix Protein MATR3.mBio. 2018 Nov 13;9(6):e02158-18. doi: 10.1128/mBio.02158-18. mBio. 2018. PMID: 30425153 Free PMC article.
-
Varicella Zoster Virus Impairs Expression of the Nonclassical Major Histocompatibility Complex Class I-Related Gene Protein (MR1).J Infect Dis. 2023 Feb 1;227(3):391-401. doi: 10.1093/infdis/jiab526. J Infect Dis. 2023. PMID: 34648018 Free PMC article.
References
-
- Anachkova, B., V. Djeliova, and G. Russev. 2005. Nuclear matrix support of DNA replication. J. Cell. Biochem. 96:951-961. - PubMed
-
- Belgrader, P., R. Dey, and R. Berezney. 1991. Molecular cloning of matrin 3. A 125-kilodalton protein of the nuclear matrix contains an extensive acidic domain. J. Biol. Chem. 266:9893-9899. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

