HLA-DP, HLA-DQ, and HLA-DR have different requirements for invariant chain and HLA-DM
- PMID: 20959457
- PMCID: PMC3003381
- DOI: 10.1074/jbc.M110.148155
HLA-DP, HLA-DQ, and HLA-DR have different requirements for invariant chain and HLA-DM
Abstract
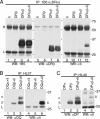
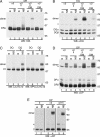
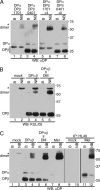
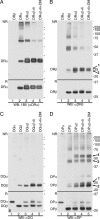
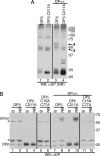
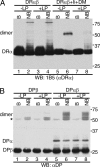
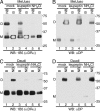
The MHC is central to the adaptive immune response. The human MHC class II is encoded by three different isotypes, HLA-DR, -DQ, and -DP, each being highly polymorphic. In contrast to HLA-DR, the intracellular assembly and trafficking of HLA-DP molecules have not been studied extensively. However, different HLA-DP variants can be either protective or risk factors for infectious diseases (e.g. hepatitis B), immune dysfunction (e.g. berylliosis), and autoimmunity (e.g. myasthenia gravis). Here, we establish a system to analyze the chaperone requirements for HLA-DP and to compare the assembly and trafficking of HLA-DP, -DQ, and -DR directly. Unlike HLA-DR1, HLA-DQ5 and HLA-DP4 can form SDS-stable dimers supported by invariant chain (Ii) in the absence of HLA-DM. Uniquely, HLA-DP also forms dimers in the presence of HLA-DM alone. In model antigen-presenting cells, SDS-stable HLA-DP complexes are resistant to treatments that prevent formation of SDS-stable HLA-DR complexes. The unexpected properties of HLA-DP molecules may help explain why they bind to a more restricted range of peptides than other human MHC class II proteins and frequently present viral peptides.
Figures
Similar articles
-
Immunochemical and functional analysis of HLA class II antigens induced by recombinant immune interferon on normal epidermal melanocytes.J Immunol. 1987 Feb 15;138(4):1310-6. J Immunol. 1987. PMID: 3100635
-
Comprehensive Analysis of CD4+ T Cell Responses to CMV pp65 Antigen Restricted by Single HLA-DR, -DQ, and -DP Allotype Within an Individual.Front Immunol. 2021 Feb 15;11:602014. doi: 10.3389/fimmu.2020.602014. eCollection 2020. Front Immunol. 2021. PMID: 33658991 Free PMC article.
-
NetMHCIIpan-3.0, a common pan-specific MHC class II prediction method including all three human MHC class II isotypes, HLA-DR, HLA-DP and HLA-DQ.Immunogenetics. 2013 Oct;65(10):711-24. doi: 10.1007/s00251-013-0720-y. Epub 2013 Jul 31. Immunogenetics. 2013. PMID: 23900783 Free PMC article.
-
How HLA-DM affects the peptide repertoire bound to HLA-DR molecules.Hum Immunol. 1997 May;54(2):170-9. doi: 10.1016/s0198-8859(97)00077-3. Hum Immunol. 1997. PMID: 9297535 Review.
-
Chronic beryllium disease: T cell recognition of a metal presented by HLA-DP.Clin Immunol. 2001 Jul;100(1):4-14. doi: 10.1006/clim.2001.5053. Clin Immunol. 2001. PMID: 11414740 Review. No abstract available.
Cited by
-
Combination vaccine based on citrullinated vimentin and enolase peptides induces potent CD4-mediated anti-tumor responses.J Immunother Cancer. 2020 Jun;8(1):e000560. doi: 10.1136/jitc-2020-000560. J Immunother Cancer. 2020. PMID: 32561639 Free PMC article.
-
A cell-based high-throughput screening assay system for inhibitor compounds of antigen presentation by HLA class II molecule.Sci Rep. 2017 Jul 28;7(1):6798. doi: 10.1038/s41598-017-07080-4. Sci Rep. 2017. PMID: 28754892 Free PMC article.
-
Pathways of antigen processing.Annu Rev Immunol. 2013;31:443-73. doi: 10.1146/annurev-immunol-032712-095910. Epub 2013 Jan 3. Annu Rev Immunol. 2013. PMID: 23298205 Free PMC article. Review.
-
HLA-DQ heterodimers in hematopoietic cell transplantation.Blood. 2022 May 19;139(20):3009-3017. doi: 10.1182/blood.2022015860. Blood. 2022. PMID: 35271697 Free PMC article.
-
Synovial fluid monocyte/macrophage subsets and their correlation to patient-reported outcomes in osteoarthritic patients: a cohort study.Arthritis Res Ther. 2019 Jan 18;21(1):26. doi: 10.1186/s13075-018-1798-2. Arthritis Res Ther. 2019. PMID: 30658702 Free PMC article.
References
-
- Hiltbold E. M., Roche P. A. (2002) Curr. Opin. Immunol. 14, 30–35 - PubMed
-
- Castelli F. A., Buhot C., Sanson A., Zarour H., Pouvelle-Moratille S., Nonn C., Gahery-Ségard H., Guillet J. G., Ménez A., Georges B., Maillère B. (2002) J. Immunol. 169, 6928–6934 - PubMed
-
- Jones E. Y., Fugger L., Strominger J. L., Siebold C. (2006) Nat. Rev. Immunol. 6, 271–282 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

