Neutralisation of interleukin-13 in mice prevents airway pathology caused by chronic exposure to house dust mite
- PMID: 20957211
- PMCID: PMC2948524
- DOI: 10.1371/journal.pone.0013136
Neutralisation of interleukin-13 in mice prevents airway pathology caused by chronic exposure to house dust mite
Abstract
Background: Repeated exposure to inhaled allergen can cause airway inflammation, remodeling and dysfunction that manifests as the symptoms of allergic asthma. We have investigated the role of the cytokine interleukin-13 (IL-13) in the generation and persistence of airway cellular inflammation, bronchial remodeling and deterioration in airway function in a model of allergic asthma caused by chronic exposure to the aeroallergen House Dust Mite (HDM).
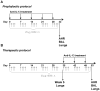
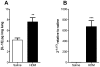
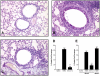
Methodology/principal findings: Mice were exposed to HDM via the intranasal route for 4 consecutive days per week for up to 8 consecutive weeks. Mice were treated either prophylactically or therapeutically with a potent neutralising anti-IL-13 monoclonal antibody (mAb) administered subcutaneously (s.c.). Airway cellular inflammation was assessed by flow cytometry, peribronchial collagen deposition by histocytochemistry and airway hyperreactivity (AHR) by invasive measurement of lung resistance (R(L)) and dynamic compliance (C(dyn)). Both prophylactic and therapeutic treatment with an anti-IL-13 mAb significantly inhibited (P<0.05) the generation and maintenance of chronic HDM-induced airway cellular inflammation, peribronchial collagen deposition, epithelial goblet cell upregulation. AHR to inhaled methacholine was reversed by prophylactic but not therapeutic treatment with anti-IL-13 mAb. Both prophylactic and therapeutic treatment with anti-IL-13 mAb significantly reversed (P<0.05) the increase in baseline R(L) and the decrease in baseline C(dyn) caused by chronic exposure to inhaled HDM.
Conclusions/significance: These data demonstrate that in a model of allergic lung disease driven by chronic exposure to a clinically relevant aeroallergen, IL-13 plays a significant role in the generation and persistence of airway inflammation, remodeling and dysfunction.
Conflict of interest statement
Figures
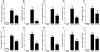

Similar articles
-
Neutralization of TSLP inhibits airway remodeling in a murine model of allergic asthma induced by chronic exposure to house dust mite.PLoS One. 2013;8(1):e51268. doi: 10.1371/journal.pone.0051268. Epub 2013 Jan 2. PLoS One. 2013. PMID: 23300949 Free PMC article.
-
Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling.Am J Respir Crit Care Med. 2004 Feb 1;169(3):378-85. doi: 10.1164/rccm.200308-1094OC. Epub 2003 Nov 3. Am J Respir Crit Care Med. 2004. PMID: 14597485
-
The interleukin-33 receptor ST2 is important for the development of peripheral airway hyperresponsiveness and inflammation in a house dust mite mouse model of asthma.Clin Exp Allergy. 2016 Mar;46(3):479-90. doi: 10.1111/cea.12683. Clin Exp Allergy. 2016. PMID: 26609909
-
IL-33 promotes airway remodeling in pediatric patients with severe steroid-resistant asthma.J Allergy Clin Immunol. 2013 Sep;132(3):676-685.e13. doi: 10.1016/j.jaci.2013.04.012. Epub 2013 Jun 4. J Allergy Clin Immunol. 2013. PMID: 23759184 Free PMC article.
-
Roles of Bronchopulmonary C-fibers in airway Hyperresponsiveness and airway remodeling induced by house dust mite.Respir Res. 2017 Nov 29;18(1):199. doi: 10.1186/s12931-017-0677-8. Respir Res. 2017. PMID: 29187212 Free PMC article.
Cited by
-
Dietary galacto-oligosaccharides prevent airway eosinophilia and hyperresponsiveness in a murine house dust mite-induced asthma model.Respir Res. 2015 Feb 7;16(1):17. doi: 10.1186/s12931-015-0171-0. Respir Res. 2015. PMID: 25849971 Free PMC article.
-
Combined administration of anti-IL-13 and anti-IL-17A at individually sub-therapeutic doses limits asthma-like symptoms in a mouse model of Th2/Th17 high asthma.Clin Exp Allergy. 2019 Mar;49(3):317-330. doi: 10.1111/cea.13301. Epub 2018 Nov 29. Clin Exp Allergy. 2019. PMID: 30353972 Free PMC article.
-
Characterization of the acute inflammatory profile and resolution of airway inflammation after Igf1r-gene targeting in a murine model of HDM-induced asthma.PLoS One. 2017 Dec 22;12(12):e0190159. doi: 10.1371/journal.pone.0190159. eCollection 2017. PLoS One. 2017. PMID: 29272313 Free PMC article.
-
Surfactant protein D attenuates sub-epithelial fibrosis in allergic airways disease through TGF-β.Respir Res. 2014 Nov 29;15(1):143. doi: 10.1186/s12931-014-0143-9. Respir Res. 2014. PMID: 25472740 Free PMC article.
-
A HuR/TGF-β1 feedback circuit regulates airway remodeling in airway smooth muscle cells.Respir Res. 2016 Sep 22;17(1):117. doi: 10.1186/s12931-016-0437-1. Respir Res. 2016. PMID: 27658983 Free PMC article.
References
-
- Willart MA, Lambrecht BN. The danger within: endogenous danger signals, atopy and asthma. Clin Exp Allergy. 2009;39:12–19. - PubMed
-
- Chiou YL, Lin CY. Der p2 activates airway smooth muscle cells in a TLR2/MyD88-dependent manner to induce an inflammatory response. J Cell Physiol. 2009;220:311–318. - PubMed
-
- Hewitt CR, Foster S, Phillips C, Horton H, Jones RM, et al. Mite allergens: significance of enzymatic activity. Allergy. 1998;53:60–63. - PubMed
-
- Johnson JR, Wiley RE, Fattouh R, Swirski FK, Gajewska BU, et al. Continuous Exposure to House Dust Mite Elicits Chronic Airway Inflammation and Structural Remodeling. Am J Respir Crit Care Med. 2004;169:378–385. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

